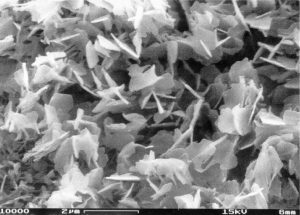
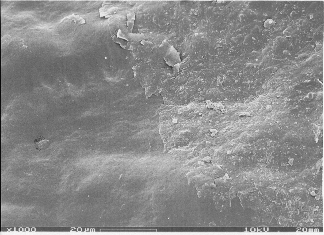
Current Projects
1. Novel wax components
 We have investigated surfaces of ca. 100 plant species and identified more than 1,000 different wax constituents (fatty acid derivatives, triterpenoids and phenolics). In the course of these analyses, we have elucidated the structures of roughly 500 novel natural products. We are currently analyzing the wax mixtures of further plant species, with special emphasis on plant families known to have broad secondary metabolite profiles. We have also started to analyze plant organs that had so far been largely neglected, for example flowers. We are using gas chromatography and mass spectrometry to separate the complex mixtures of plant waxes and identify their constituents. Microscale syntheses of authentic standards are carried out where necessary to confirm structures after they had been assigned by spectroscopy.
We have investigated surfaces of ca. 100 plant species and identified more than 1,000 different wax constituents (fatty acid derivatives, triterpenoids and phenolics). In the course of these analyses, we have elucidated the structures of roughly 500 novel natural products. We are currently analyzing the wax mixtures of further plant species, with special emphasis on plant families known to have broad secondary metabolite profiles. We have also started to analyze plant organs that had so far been largely neglected, for example flowers. We are using gas chromatography and mass spectrometry to separate the complex mixtures of plant waxes and identify their constituents. Microscale syntheses of authentic standards are carried out where necessary to confirm structures after they had been assigned by spectroscopy.
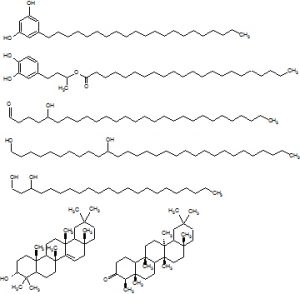
Amongst others, we have identified homologous series of delta-lactones, hydroxyaldehydes, and alkanediols. These compound classes were found to share the special arrangement of two functional groups in 1,3-, 1,5-, 1,7-, 1,9-, or 1,11-positions. We therefore hypothesize that these compounds are polyketides, and that their biosynthesis proceeds via special elongation pathways. This idea is currently being tested by experiments involving the cloning and characterization of relevant candidate enzymes.
2. Chemical patterns on plant surfaces
 We could, for the first time, selectively prepare the outermost portion of surface waxes for quantitative analysis. For several plant species the outer wax layer was less than 1 μm thick (less than 50 layers of molecules), and its chemical composition differed from that of the underlying wax mixture. The surfaces of some plant species additionally show microscopic crystals with characteristic shapes and arrangements. We developed methods to sample these wax crystals for direct analysis. We then characterized new crystal types based on their major constituents, and confirmed that these constituents can form identical structures in vitro. These methods are currently used to investigate the layered structure of the skin waxes of more plant species.
We could, for the first time, selectively prepare the outermost portion of surface waxes for quantitative analysis. For several plant species the outer wax layer was less than 1 μm thick (less than 50 layers of molecules), and its chemical composition differed from that of the underlying wax mixture. The surfaces of some plant species additionally show microscopic crystals with characteristic shapes and arrangements. We developed methods to sample these wax crystals for direct analysis. We then characterized new crystal types based on their major constituents, and confirmed that these constituents can form identical structures in vitro. These methods are currently used to investigate the layered structure of the skin waxes of more plant species.
 We have also used various microspectroscopic techniques to directly map the chemical composition of the plant surface. In collaboration with Mike Blades and Robin Turner (both UBC), we have used confocal Raman spectroscopy to investigate the lateral distribution of triterpenoids on the surface of Prunus laurocerasus leaves. We found that these cyclic compounds were accumulating largely in the cuticle areas above periclinal cell walls, whereas the long-chain wax constituents were evenly distributed across periclinal and anticlinal regions.
We have also used various microspectroscopic techniques to directly map the chemical composition of the plant surface. In collaboration with Mike Blades and Robin Turner (both UBC), we have used confocal Raman spectroscopy to investigate the lateral distribution of triterpenoids on the surface of Prunus laurocerasus leaves. We found that these cyclic compounds were accumulating largely in the cuticle areas above periclinal cell walls, whereas the long-chain wax constituents were evenly distributed across periclinal and anticlinal regions.
We further used ToF-SIMS (Time of Flight Secondary Ion Mass Spectrometry), a technique with spatial resolution in the nm range, to further map plant surface chemistry. In a first study, together with Rana Sodhi (UofT), we have investigated leaves of Kalanchoe daigremontiana, showing that triterpenoids are evenly distributed on the surface in this case.
map plant surface chemistry. In a first study, together with Rana Sodhi (UofT), we have investigated leaves of Kalanchoe daigremontiana, showing that triterpenoids are evenly distributed on the surface in this case.
3. Wax biosynthesis in Arabidopsis thaliana
Very-long-chain compounds
We are using Arabidopsis thaliana as a model system to elucidate the biosynthetic pathways leading to various wax components. My group has led the investigations of MAH1 (for Midchain Alkane Hydroxylase), a cytochrome P450-dependent enzyme catalyzing the hydroxylation of very-long-chain alkanes, with high region-specificity forming secondary alcohols, ketones and ketols. MAH1 is thus the enzyme catalyzing the final steps along (one branch of) the wax biosynthetic pathway. In this context, it is important to note that MAH1 was localized to the endoplasmic reticulum together with all other wax biosynthetic proteins, showing that the wax biosynthesis machinery is entirely confined to the ER of epidermal cells.
My group has isolated and characterized various other genes involved in the biosynthesis of very-long-chain wax compounds in Arabidopsis and other plant species such as tomato, rye and wheat. Currently, we are investigating the molecular genetics and protein biochemistry underlying the formation of diverse fatty acid derivatives in these and other species. Ultimately, we aim to establish the tools for optimizing the wax mixtures on various crops to enhance their drought resistance.
Triterpenoids
Characteristic triterpenoids had repeatedly been reported in cuticular wax mixtures of selected plant species, organs or developmental stages, frequently accumulating to only a few percent of the wax mixture and sometimes only as trace components. In some instances their role as anti-feedants against insects and vertebrates was discussed. However, it was not clear whether these secondary metabolites were indeed biosynthesized for a targeted, and possibly even sole function in the plant cuticle. In other plant species the stem surface is slippery due to the presence of thread-like crystals consisting of triterpenoids (link to below). In these cases it is plausible that the triterpenoids are made for the cuticle, but it remains open whether they perform other functions elsewhere as well. In order to answer these questions, my lab has launched investigations into the biosynthesis of cuticular triterpenoids.
It is well established that the biosynthesis of plant triterpenoids proceeds via a highly stereoselective cyclisation reaction, yielding either cycloartenol (steroid biosynthesis) or triterpenols with characteristic carbon skeletons. We have used a PCR-based approach to clone homologous gene products from selected plant systems, including Castor bean, tomato and Kalanchoe daigremontiana. The product spectra of the enzyme products were investigated in vivo using heterologous expression in yeast. Finally, we have analyzed the expression patterns of the genes to test their involvement in the biosynthesis of cuticular wax. We found that between one and four different cyclase enzymes are present in the different plant species investigated, together accounting for the triterpenoid composition of corresponding cuticular waxes. All these enzymes were expressed only in the surface tissues of the plants, strongly suggesting that they only function to produce cuticular triterpenoids. Thus, we conclude that the sole raison-d’être of cuticular triterpenoids is indeed to moderate the wax properties and aid in the diverse eco-physiological functions of the plant surface.
4. Ecophysiological functions of the plant surface
Transpiration barrier
It has long been known that the primary function of plant cuticular waxes is to restrict non-stomatal water loss. However, it is still not clear how the different components of the wax mixture contribute to this function. Why do plants make such complex wax mixtures that have very tightly-controlled composition for each species yet they differ so drastically between species? Many attempts have been made at correlating the water barrier properties of cuticles with their wax mixture composition, but no structure-function relationships could be established so far. It is of crucial importance to understand the causal link between composition and physiological function in order to develop future crop lines that have improved drought resistance through enhanced cuticular transpiration barrier properties.
In one project, my lab has adapted methods developed by Joerg Schoenherr, Markus Riederer and Lukas Schreiber to measure the cuticular barrier properties on various plant organs spanning a wide range of water permeabilities. In a first model study, we have investigated the cuticular transpiration barrier on petals of Cosmos bipinnatus, and found that they are significantly more permeable than most of the leaf and fruit cuticles investigated to date. We are currently modifying the methods for further use on materials with artificially altered wax compositions, to test possible structure-function relationships.
Plant-ant interactions
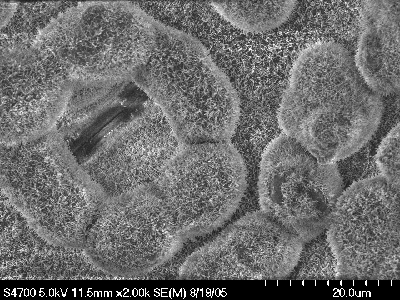
The stems of many Macaranga ant-plants (Euphorbiaceae) are densely covered with microscopic wax crystals. Symbiotic ant species can adhere to and walk on these vertical surfaces. In contrast, the crystals are slippery for generalist insect species and they are excluded from higher parts of the plants. In this system surface wax crystals consequently function as a mechanical barrier that protects specialist Macaranga partners against competition by other ant species.
My lab has carried out detailed chemical analyses of the cuticular crystals that make the Macaranga surfaces slippery. We found that various triterpenoid compounds, differing in structure between different Macaranga species, are responsible for surface crystal formation. Based on these chemical results, my lab has also launched investigations into the biosynthetic machinery needed to generate the high quantities of cuticular triterpenoids. We have begun to clone relevant genes, and are currently characterizing the encoded proteins by heterologous expression in yeast.
Our long-term goal is to understand the mechanisms causing the slipperiness of wax crystals for walking insects. The Macaranga project provides one example where diverse ant species and diverse plant partners can be compared, assessing both the physical properties of the plant surfaces and the corresponding chemical composition. Based on these data, model surfaces with corresponding characteristics will be prepared and further tested. In this project, we collaborate with Walter Federle (University of Cambridge), who is investigating the adhesive organs and the processes of movement both of specialist Macaranga partner ants and their generalist competitors.
Slippery surfaces on carnivorous plants
Plants in the carnivorous genus Nepenthes obtain a substantial nutrient supply by trapping insects in highly modified leaves. A broad zone of the inner surface of these pitchers is densely covered with wax crystals on which most insects lose their footing. This slippery wax surface, capturing prey and preventing its escape from the trap, plays a pivotal role in the carnivorous syndrome. To understand the mechanism of slipperiness, we have started to characterize the ultrastructure and the physico-chemical properties of the wax crystals in pitchers of various Nepenthes species.
 Scanning electron microscopy has revealed that characteristic wax platelets protrude perpendicularly from the surface. My lab has developed methods that allow the mechanical removal of these wax crystals from the pitcher surface. We have shown that the sampling was selective for the outermost part of the wax crystals, relevant for plant-insect-interactions, and can now for the first time directly assess the quantities of all compounds involved in crystal formation. GC-MS investigation of the wax extracts showed that the crystals are mainly formed by the C30 aldehyde triacontanal. However, further studies by ATR-FTIR provided indirect evidence that the crystals actually contain oligomeric or polymeric acetals of this aldehyde. We are planning to further characterize the molecular structure of the slippery wax crystals, and also their biomechanical properties.
Scanning electron microscopy has revealed that characteristic wax platelets protrude perpendicularly from the surface. My lab has developed methods that allow the mechanical removal of these wax crystals from the pitcher surface. We have shown that the sampling was selective for the outermost part of the wax crystals, relevant for plant-insect-interactions, and can now for the first time directly assess the quantities of all compounds involved in crystal formation. GC-MS investigation of the wax extracts showed that the crystals are mainly formed by the C30 aldehyde triacontanal. However, further studies by ATR-FTIR provided indirect evidence that the crystals actually contain oligomeric or polymeric acetals of this aldehyde. We are planning to further characterize the molecular structure of the slippery wax crystals, and also their biomechanical properties.