Want to learn about the different gases in the atmosphere and how they affect the earth? CHEM 302 is a Chemistry course that discusses reactions that describe environmental phenomena such as the ozone layer, pollution, and ozone holes.
format of the course
The format of the course is pretty standard. There were live lectures with quizzes, a midterm, and a final exam. Quizzes were fairly easy with unlimited attempts for each; however, you did not get to know the correct answers till after the submission deadline. Since I took this course virtually, both the midterm and final exam were quizzes on Canvas. The midterm was straightforward and easy, as the concepts of the first half of the course were not complex at all. The final exam was pretty difficult, due to the various reaction mechanisms that you had to memorize. To supplement your studying, there were practice problem sets (not for marks) that contained difficult problems slightly above exam difficulty.
gpa 🙂 or 🙁
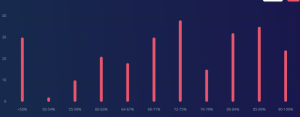
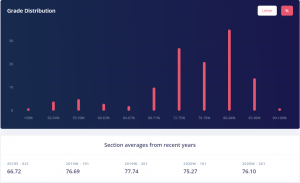
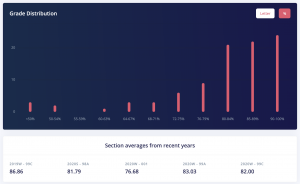
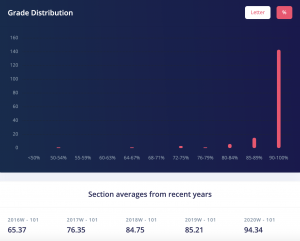
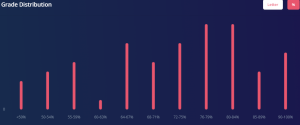
This is 100% a GPA booster as a lot of the content is review from 1st year Chemistry. The averages for quizzes were high 90s and for the exams they were low to mid 80s. If you put in the work this is an easy A or A+, as the class average for when I took it was 83. You can also see from the grade distribution that most people achieved an A+.

CHEM 302 Grade Distribution. Credits: ubcgrades.com
verdict: to take or not to take
If you are in Chemistry and want a break in your schedule, I would definitely take this course. I had the advantage of being in Chemical Biology, thus a lot of the Chemistry concepts in this course were basically review from 1st and 2nd year. However, this course is entirely doable for life science majors, as the Chemistry concepts are super easy to follow.