When we think of water, we usually think of it having three states: solid, liquid, and gaseous. Water is a liquid between 0°C and 100°C (32°F and 212°F) under our standard atmospheric pressure of 1 atmosphere (760 Torr, 14.7 psi, 1.013 bar). Above 100°C, water becomes a gas (vapour), and below 0°C, water becomes a solid (ice). The pressure also affects the temperature at which the phase change occurs: in a pressure cooker, the temperature can reach 121°C with an additional 1 bar of pressure (0.987 atm, 750 Torr, 14.5 psi) before boiling. In a freeze dryer, the vacuum reduces the pressure as low as 0.1 mbar (9.87 x 10^-5 atm, 75 mTorr, 0.00145 psi), where water can boil at almost -40°C. This information can be displayed in a graph called a phase diagram, displaying the phase of the substance at a given pressure and temperature.

Phase diagram of water. Credit: Wikimedia
However, there are multiple possible crystal structures the water molecules can arrange themselves into, with different forms being favoured at different temperatures and pressures, and with different stabilities and ordering. This can be shown in a more complex phase diagram.
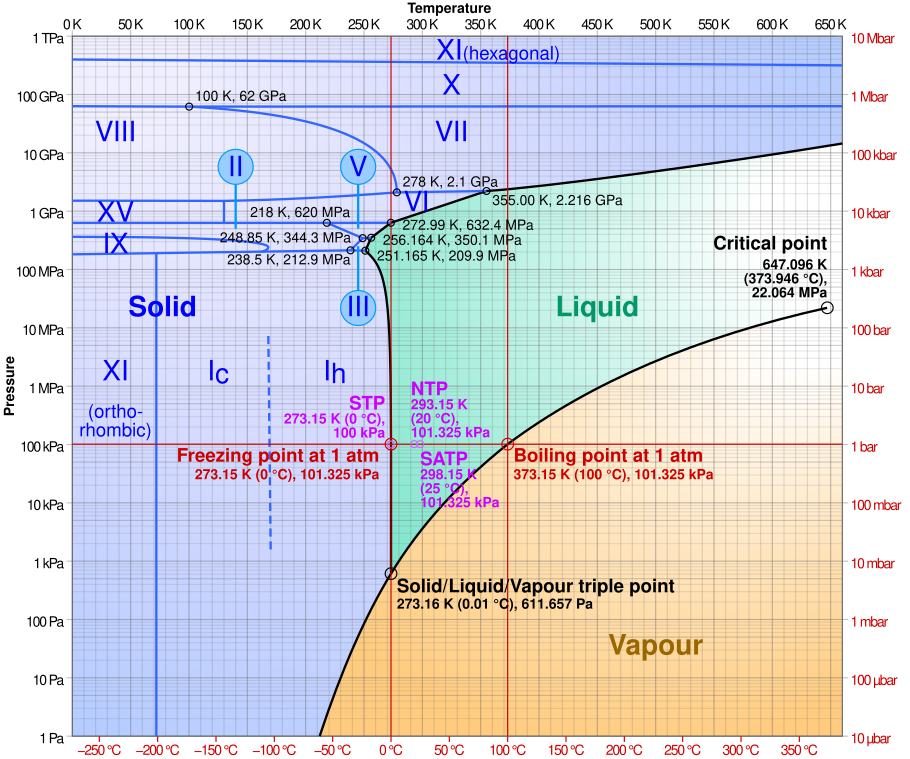
Phase diagram of water, with multiple phases of ice included. Credit: Wikimedia
Most of the ice on earth is in the ice I phase, specifically ice Ih, which is the hexagonal form of ice that we encounter in everyday life. As of 2021, there are now twenty known crystalline forms of ice (Hansen, 2021) with scientists still attempting to discover more. This results in a very complicated phase diagram of different phases of ice (Fig 1 in link).
In addition to crystalline ice, there is also amorphous ice. Amorphous solids do not have a defined crystal structure – one example in daily life is glass. Hence, amorphous ice is often described as being “glassy” or “vitreous”. So far, three forms of amorphous ice have been identified (Martoňáka et al., 2005). All these forms of ice have distinct properties, as well.
Even though water may seem simple, there is still plenty of ongoing research on the peculiarities of water and its properties. Scientists speculate that there may be further forms of ice that have yet to be discovered… perhaps you will join the search?
