Glow in the dark toys. Glow sticks. They both glow, but not in the same way… So what’s the difference? Luminescence, phosphorescence, fluorescence… what does all of this mean?
Luminescence refers to the process where light is emitted from an object that is not due to the object’s temperature. This contrasts with incandescence – the process by which hot metal or a fire glows. With incandescent objects, the colour of the light is related to the temperature of the object.

Colour of radiation from a black body with increasing temperature (in Kelvin). Credits: Wikimedia
There are many forms of luminescence as well. For instance, glow sticks exhibit chemiluminescence: chemical energy is converted into visible light through the excitation and subsequent relaxation of electrons in a molecule – more on that here. Another form of luminescence is triboluminescence, which is a form of mechanoluminescence – mechanical energy is converted to light. You may be familiar with this from demonstrations of crushing sugar cubes, putting on bandages, or peeling off tape in the dark.
Glow sticks. Credits: Wikimedia
Triboluminescence of nicotine salicylate. Credits: Wikimedia
There is also photoluminescence, where higher energy photons are absorbed and lower energy photons are emitted. Fluorescence and phosphorescence are in this category. In fluorescence, the light is absorbed and re-emitted almost immediately – this would include fluorescent molecules such as those in fluorescent markers, luminol, quinine in tonic water, and riboflavin or vitamin B2. These are often viewed under UV light, which contains high energy photons that excite molecules, emitting lower energy photons. In phosphorescence, the photons are emitted over a longer period of time. This is the form of luminescence found in glow-in-the-dark dinosaur toys.

Fluorescence of quinine in tonic water. Credits: Wikimedia

Glow-in-the-dark figure. Credits: Wikimedia
The difference between fluorescence and luminescence can be visualized in a Jablonski diagram.
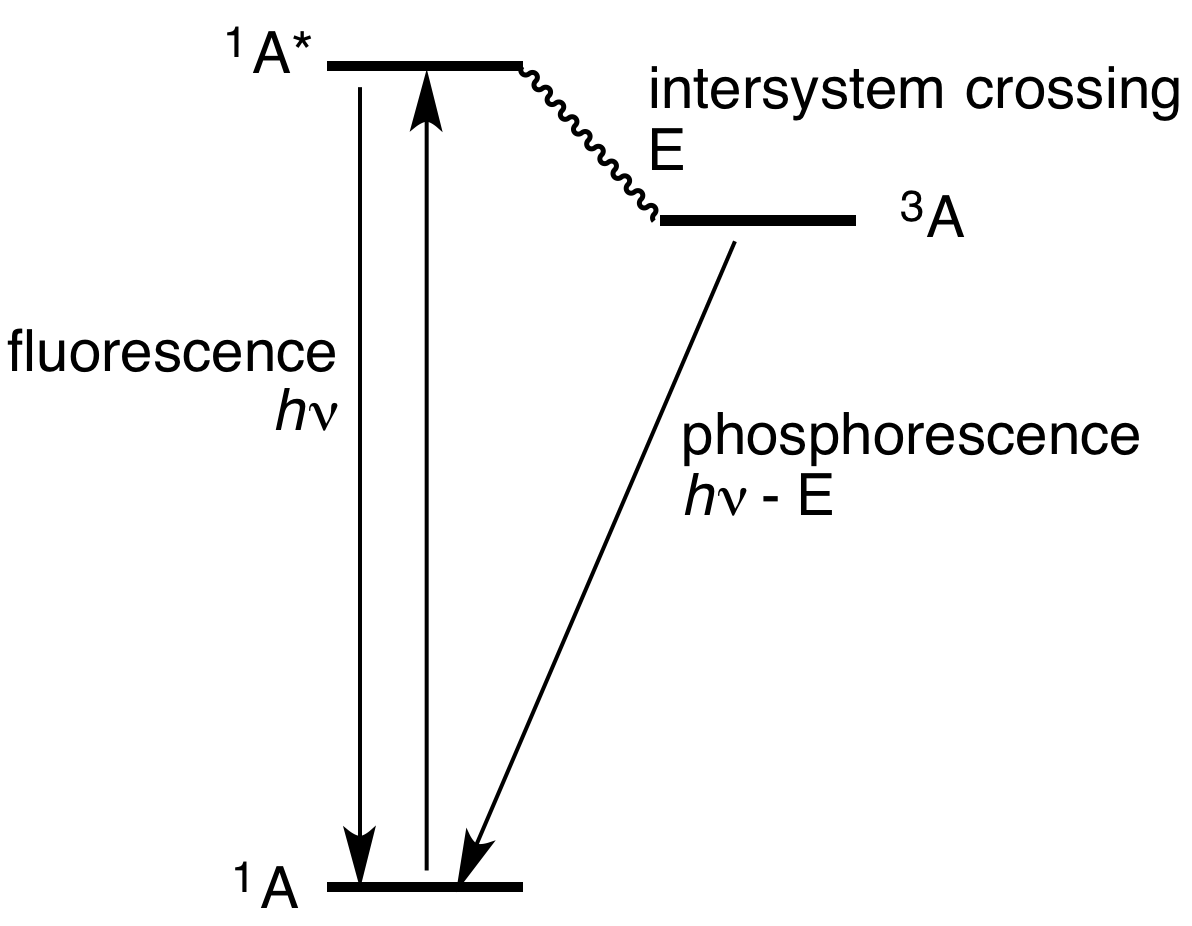
Jablonski diagram comparing fluorescence and phosphorescence. Credits: Wikimedia
This shows a molecule in its ground singlet state (1A), which is at its lowest energy level and has no unpaired electrons. With an input of energy, it goes into an excited singlet state (1A*). From here, there is vibrational relaxation down to a lower energy state (not pictured). If the energy is emitted from this state, it is called fluorescence. However, it is also possible (although less likely) for the molecule to transition into a triplet state with unpaired electrons through intersystem crossing. The triplet state (3A) lasts longer than the excited singlet state, and so the relaxation and emission of energy as light takes longer, resulting in the lasting glow.
