Let’s face it, to most people gold is just an over-glorified rock with no real value; however, that’s not the case at all! Just this month, researchers from University College London have created a novel light-activated coating that kills infectious bacteria. The key ingredient? Gold.
upgrading with gold…
The invention of a bacteria-killing coating sounds ingenious; however, Hwang’s team was actually not the first to come up with this idea. Previous studies have already shown that coatings incorporating the chemical crystal violet can adequately kill bacteria. The problem was that the coating had to be light-activated by UV rays, which harm the skin by promoting skin cancer.
This was exactly the problem Hwang’s team looked to solve; to make a coating that did not require harmful wavelengths of light. They overcame this challenge by incorporating small clusters of gold into a polymer containing crystal violet. The result? Now this new coating could effectively eliminate bacteria upon activation with low intensity white light – the level of light found in offices.
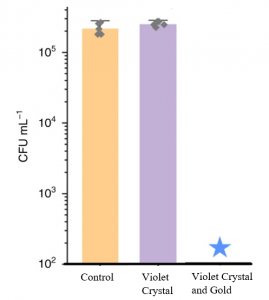
Concentration of bacteria (CFU/mL) across three conditions after 6 hours exposure to low-intensity white light. Star indicates bacterial concentration is undetected. Sample size = 6 per treatment, error bars are standard deviation. Adapted from Hwang et al.’s data
The figure above perfectly illustrates their result. Statistical analyses show that bacterial concentration does not significantly differ between the violet crystal and control (no coating) condition. This indicates that low-intensity white light cannot activate the bacterial-killing function in the violet crystal coating. What’s interesting is that addition of gold with the violet crystal, reduces the bacterial concentration significantly to near zero values, indicating successful activation.
More than a novelty…
The results of Hwang’s study are truly impactful. It is well known that hospitals are a hotbed for infectious bacteria. In fact, 27% of surfaces in hospital rooms are contaminated with bacteria even after regular and thorough cleaning. As such, applying the coating on these surfaces will definitely reduce the chances of contracting a hospital-related disease. Who would have thought? Not only is gold more than just a hunk of rock, it can also save lives.