The process of protein synthesis has probably been ingrained in your brain if you have taken any introductory biology or cell biology courses. It is so important that it is often referred to as the central dogma of molecular biology: DNA is transcribed to RNA, which is then translated to proteins.
Proteins essentially carry out the functions needed for the cells to remain alive. Need something made or broken down? Enzymes. Need something transported or moved? Carrier and intramembrane proteins. When it comes to proteins, shape equals function.
With their vast variety in function, the way a protein folds as it is made is closely monitored. Protein folding is faster than translation, thus the moment the N-terminal is exposed to the aqueous environment of the cytosol, the chain begins to fold due to intramolecular forces. As the chain continues to elongate, the protein can become kinetically trapped because the native state of a protein is only partially stable. Partially folded or misfolded states are problematic because they tend to aggregate due to their exposed hydrophobic surfaces (Hartl, F., et al. 2011).
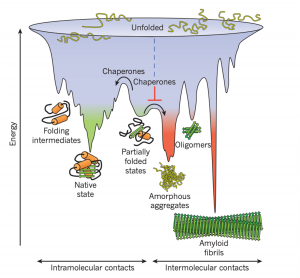
Figure 1: Competing reactions of protein folding and aggregation (Hartl, F., et al. 2011)
So, what to do with a misfolded protein? Chaperones and chaperonins are in charge or re-folding them. Also known as heat-shock proteins (hsp’s). These proteins seek and bind to exposed hydrophobic surfaces in newly translated proteins (Campbell, M., & Farrel, S. 2012).
Chaperones (Hsp70) bind and stabilize misfolded or partially folded proteins and prevent their aggregation as they are translated. ATP binding causes the chaperone to release the completed chain into the cytosol, allowing the protein to fold properly (Albert, B. 2015).
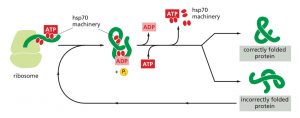
Figure 2: The hsp70 family of molecular chaperones (Albert, B. 2015)
Sometimes, even the chaperones are unsuccessful in helping the protein re-fold into its native state. In this case, additional chaperones or the more complex chaperonins (hsp60 and hsp10) provide additional help.
Chaperonins create a chamber for the proteins to re-fold post-translationally. First, the protein is captured though hydrophobic interactions with the entrance of the chamber (hsp60). The protein is then released into the interior of the chamber, which is lined with hydrophilic amino acids, and then it is sealed with a lid (hsp10). Here, the substrate can fold into its final conformation in isolation, where there are no other proteins that may aggregate. Finally, when ATP is hydrolyzed, the lid pops off, and the substrate protein is released from the chamber (Albert, B. 2015).
The best characterized chaperonin is the GroEL/GroES system in E. coli

Figure 3: Visualization of the GroEL/GroES chaperonins in E. coli (Rachel Davidovitz, 2014)
If the protein is still improperly folded, it is then targeted for degradation (ubiquitination, we’ll talk about it next time!)
References:
Albert, B, et al. (2015). Molecular Biology of the Cell (6th Edition). Garland Science, Taylor & Francis Group, LLC.
Campbell, M., & Farrel, S. (2012). Biochemistry (7th Edition). Brooks/Cole Cengage Learning.
Hartl, F. U., Bracher, A., & Hayer-Hartl, M. (2011). Molecular chaperones in protein folding and proteostasis. Nature, 475(7356), 324-332.doi:10.1038/nature10317
Rachel Davidovitz (2014, October 6). Active Cage Mechanism of Chaperonin-Assisted Protein Folding Demonstrated at Single-Molecule Level [Video]. https://www.youtube.com/watch?v=–NcNeLc1mo&ab_channel=RachelDavidowitz