Everyone has heard that too much stress will cause grey hair. This is easily seen in former president of the United States, Barack Obama, whose hair could not escape the stress of the Oval Office! But what exactly links grey hair and stress? This year, researchers at Harvard University found that the nervous system eliminates pigment-regenerating stem cells responsible for coloring our hair!

Barack Obama’s hair color at the start of his presidency versus seven years after. Credits: DailyMail.com
THE ROOT OF THE PROBLEM
When you are stressed, your body responds in three distinct ways: the activation of your immune system, the activation of your sympathetic nervous system (SNS), and the release of cortisol, an energy-stimulating hormone. All these responses put your body into a “fight or flight” mode; increasing heart rate and blood pressure. The challenge for Zhang’s team was to sort through these three responses and determine which caused grey hair.
Zhang’s team tackled this problem by performing a series of experiments on black-furred mice. They first tested if immune system activation was the cause by seeing if the fur greyed under stress, even when the immune system was deactivated. They indeed found that stressed immune-deficient mice still greyed, indicating that stress causes greying, independent of an immune response.
They also ran similar experiments using mice mutated to not respond to cortisol or noradrenaline, a molecule involved in SNS activation. The idea being that if a response was involved, stress should not cause the fur to grey if it was removed. In mice lacking response to cortisol, the fur still greyed; however, in mice lacking the response to noradrenaline, their fur remained black! This indicated that the SNS was the main driver in hair greying.
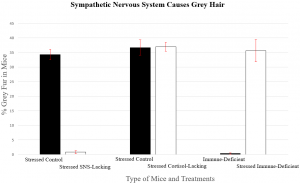
Figure 1. The results of the experiments described above are shown. Note that mice unable to respond to SNS activation do not grey under stress. “Control” refers to unmutated mice. Also note that a different type of control (non-stressed vs stressed) was ran in the immune-deficient case. (Sample size = 6 for each condition, standard error bars). Credits: Adapted from Zhang et al.’s data.
ZOOMING IN FURTHER…
With the culprit in hand, Zhang’s team didn’t just stop there! Through further experimentation, they illustrated that the SNS over-stimulates MeSC, the stem cells involved with hair pigmentation. During hair growth, these MeSC cells transform into pigment-producing cells and color the hair. Under stress, the SNS causes these MeSC cells to transform at an abnormally high rate, quickly depleting these cells and leading to grey hair.
THE REASON BEHIND THIS LINK?
In truth, the reason why this MeSC and SNS interaction exists is unclear. Zhang’s team suggests an evolutionary perspective. Since octopuses, a distant relative to mice and humans, can modify pigmentation of their skin using the SNS, they hypothesize that this interaction was simply conserved. Whatever the reasons may be, this just further shows that the mystery has yet to be completely solved!