A potentially fun course ruined by poor administration and lack of communication. CHEM 205 focuses on the fundamentals of thermodynamics, kinetics, and spectroscopy useful for life science students.
format of the course
Lectures consisted of a professor going through a slide deck, as well as in-class practice problems. Conceptually the material was quite interesting, however the derivations for different equations can be quite dry to listen to. There were homework questions which weren’t incredibly challenging, but doing the math can become quite tedious.
gpa 🙂 or 🙁
This is a GPA booster if you’re good at math, otherwise it’s pretty GPA neutral. Personally, I found the evaluations to be ridiculously unfair. For the final exam, it was an EXACT (WORD FOR WORD) copy of a past 2013 final exam. Although this final exam was not released formally, the solutions could be found on CourseHero. Basically, if you had access to this exam beforehand you were getting an A+.
What’s more off-putting is that several individuals on Piazza actually defended viewing the exam beforehand. Their reasoning being that everyone who didn’t have access were all idiots for not taking advantage of all their resources (we now know in these COVID times – Chegg and coursehero is tantamount to academic dishonesty).
Unfortunately, even before the final exam – these same individuals were stirring trouble within the class. After a particularly difficult midterm, they were taunting others telling them that “you wont make medical school if you didn’t get 100 on this midterm” – really living up to the toxic premed stereotype (funny thing being that noone brought up the subject of medical school either … how they correlated performance in a physical chemistry course to medical school admissions is beyond me).
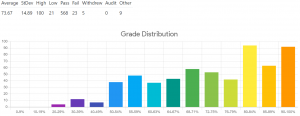
CHEM 205 grade distribution. Credits: ubcgrades.com
verdict? to take or not to take
Considering my toxic experience with my course I cannot recommend this to anyone. In fact, I don’t think things have gotten any better.
