Ever wonder how evidence is processed from a crime scene?
In the 19th century, British chemist James Marsh first developed a forensic method for detecting arsenic. And just in a period of ten years, the Marsh test solved 98 poison related cases all over England and Wales. Since then, forensic chemistry has always been an important aspect in a criminal investigation.

Source: Wikimedia
Nowadays, different substances can be identified using familiar laboratory instruments such as Fourier Transform Infrared Spectroscopy, Thin Layer Chromatography, High Performance Liquid Chromatography, and more. These identifications play a crucial part in a police investigation as they provide investigators with leads suggesting how a crime is committed.
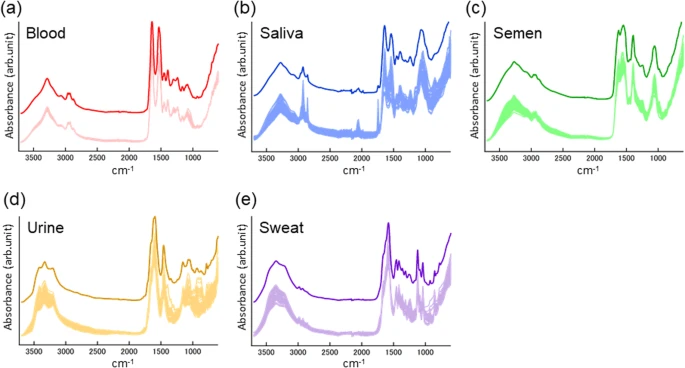
Different body fluids detection through FTIR. Source: Ayari Takamura and others
Remember that scene in the movie where a crime scene investigator sprays chemical onto something, and then trace of blood becomes apparent? This is called Luminol test. In the presence of blood, luminol reacts by fluorescing, thus emitting a blue glow in a dark room. Luminol is a water-based solution that detects blood even after it has been diluted 10,000 times.
The Kastle–Meyer test is another blood detection test that is common in crime labs. Phenolphthalein reacts with hydrogen peroxide in the presence of hemoglobin to turn from colorless to pink.
One important branch of forensic science is toxicology. A toxicology test uses principle of analytical chemistry , biology, and the study of poisons to determine the identity and relative amount of substances presence in one’s body fluids. A tox screen can provide the police with valuable information to the nature of the investigation, such as poisoning, overdose, suicidal or homicidal.
A forensic scientist is Sherlock with a microscope!
Really enjoyed reading this piece and about how far forensic science has come since the days of James Marsh. I wonder what role the jury has in the interpretation and credibility of forensic tests when used in court? I liked your use of narrative style which led me from the beginnings of forensic science to the present day!
I really enjoyed this blog! I liked the hook question at the beginning and the immediate explanation; it kept me reading. I am excited to hear about more field methods that don’t require large non-portable machines (for example, HPLC).
Good use of narrative style. However, the graphs have two curves, and it wasn’t clear what each curve shows. (I assumed the lower curve illustrates normal levels)