Are you thinking about wearing coloured contact lenses to change the colour of your eyes? While it may appear to be a simple and risk-free way to enhance your natural eye colour or make a fashion statement, it is critical to consider the chemical composition as well as the potential risks associated with these lenses. Let’s look at the chemistry of coloured contact lenses and weigh the upsides and downsides.

Better Vision different colour contact lenses. Source.
Reasons Against Coloured Contact Lenses:
- They Increase the Risk of Eye Infections: Contact lenses are made of hydrophilic polymers, which attract and retain water. This makes them an ideal surface for microorganisms to grow on, increasing the risk of eye infections. If you do not clean and disinfect your coloured contact lenses properly, you may increase your risk.
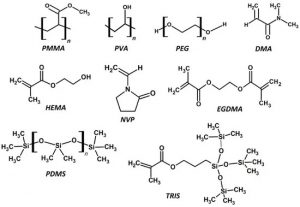
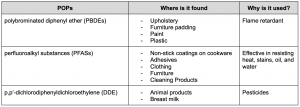
Common monomers and polymers used in contact lens production. PMMA—poly methyl methacrylate, PVA—poly vinyl alcohol, PEG—poly ethylene glycol, DMA—dimethyl methacrylate, HEMA—hydroxy ethyl methacrylate, NVP—N-vinyl pyrrolidone, EGDMA—ethylene glycol dimethacrylate, PDMS—poly dimethyl siloxane, TRIS—3-[tris(trimethylsiloxy)silyl]propyl methacrylate. Source
- Manufacturers Might Not Follow Strict Safety Standards: Coloured contact lenses are frequently regarded as cosmetic devices and are therefore not subject to the same regulations as medical devices. Some manufacturers may fail to adhere to strict safety standards, resulting in poorly made lenses that harm your eyes. It is critical to select a reputable brand and avoid purchasing lenses from untrustworthy sources.
- Sharing Coloured Contact Lenses is a Big No-No: Sharing coloured contact lenses or wearing them for an extended period of time can increase the risk of eye infection. This is because the lenses can harbour bacteria, which can cause long-term damage to your eyes. It is critical to carefully follow the instructions and avoid sharing your lenses with anyone.
Reasons in Favour of Coloured Contact Lenses:

Different coloured contact lenses in the same individual. Source.
- They Allow Personal Expression: Coloured contact lenses are a fun and versatile way to express yourself and your sense of style. You can switch up your look as often as you like because there are so many different colours and designs to choose from. This is especially appealing to those who do not want to commit to long-term solutions such as cosmetic surgery or tattoos.
- They Are Made of Safe Materials: Coloured contact lenses are made of eye-safe materials such as silicone hydrogels or methacrylate-based polymers. These materials are designed to be biocompatible, which means they will not harm or irritate your eyes.
- They Can Be Customized to Your Needs: Coloured contact lenses are available in prescription and non-prescription forms, allowing you to tailor them to your specific requirements. This is especially beneficial for people who need vision correction but want to experiment with different eye colours.
Finally, while coloured contact lenses can be a fun and exciting way to experiment with your personal style, it is critical to prioritize your eye health and safety. You can reduce your risk of eye infections and enjoy the benefits of coloured contacts by selecting a reputable brand, following proper cleaning procedures, and avoiding sharing your lenses. Whether you’re looking to enhance your natural eye colour or completely change your look, remember to prioritize your eye health and enjoy your new look with confidence!
~ Vivian Hou