Chemists from the University of California, Berkley (UCB) have designed a new material that could reduce the energy requirements of the Haber-Bosch process. The group hopes their research, published January 11th 2023, will conserve energy and lead to a “greener” future for ammonia and fertilizer production.

Current infrastructure needed to maintain the pressure and temperature required for the Haber-Bosch process source
The Haber-Bosch process has been the main method for producing ammonia since its invention over 100 years ago. It is widely considered one of the most important scientific discoveries of the 20th century. Yet, despite its important role producing ammonia for agricultural fertilizer, its industrial synthesis continues to be energy inefficient.
High temperatures and pressures are needed to produce ammonia which must then be extracted to be used. Conventionally, the reaction mixture is cooled from 500℃ to -20℃. This condenses the synthesized ammonia and separates it from the remaining chemicals. However, cooling the mixture while maintaining the pressure of 300 atmospheres accounts for a large proportion of the processes’ energy loss.
Benjamin Snyder, who leads the UCB research group, said it was this extraction step that his team sought to improve by “finding a material where you can capture and then release very large quantities of ammonia, ideally with a minimal input of energy”.
These requirements led the research group to create a metal-organic framework (MOF) material. The MOF had a crystal structure made from copper atoms linked to cyclohexane dicarboxylate molecules. The crystalline structure gave the material unique properties suited for its use in ammonia extraction.
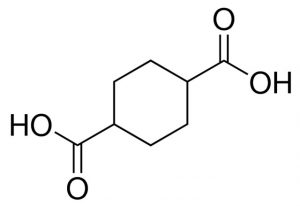
Structure of the cyclohexane dicarboxylate molecule used to make the MOF source
When exposed to ammonia the material changes its structure from a rigid crystal to a loosely packed and porous polymer. The polymer form can readily store a large amount of ammonia within it which can then be released with cooling. The result is that ammonia can be extracted 195℃ above the temperature required by current methods and at half the pressure.
Not only would the MOF save energy in the extraction process but, interestingly, after releasing the ammonia “the polymer somehow weaves itself back into a three-dimensional framework” says Snyder. This mechanism, which is still being studied, allows the MOF to be used repeatably.
With the Haber-Bosch process using 1% of the world’s energy, the research done by Snyder and his group is an important step in producing a greener future for ammonia.