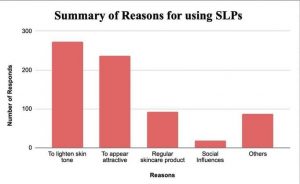
Skin colour has long been the social-economic benchmark in many countries, predominantly in Asia-Pacific, where individuals often affiliate lighter complexions with attractiveness and more career opportunities. Skin lightening products (SLPs), the practice of lightening complexion by reducing the skin’s natural pigments, is often the solution to this dilemma. This growing demand created the lucrative business of skin-lightening products (SLPs), which was valued at US$8.8 billion in 2020.
Skin beauty is very important to many people by enhancing users’ self-esteem and confidence. Oftentimes, people reported wanting a lighter, radiant, and youthful skin, an indicative symbol of beauty and youth.
Some active ingredients in SLPs shown results in correcting the effects of age, stress, UV exposure, pollution, and poor eating. They also help maintain a luminous skin by preventing skin dryness, reducing oxidative damage, and balancing skin tone. SLPs coat the skin’s surface and act as a protection layer, thereby preventing issues like tanning and sunspots.
For most people, the utility that SLPs offer are essential to maintaining their beauty and confidence.
However, a major concern when it comes with SLPs is the uncontrolled concentrations of these active ingredients and their negative side effects.
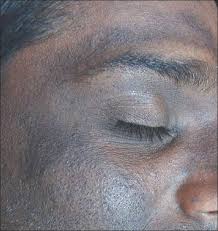
Hydroquinone, a potent SL agent for hyperpigmentation treatments, causes blue-black facial discoloration (Figure 1) or skin thinning with high-dosage applications, said Dr. Desai, a board-certified dermatologist at the University of Texas Southwestern Medical Center. This condition is hard to treat, and can result in permanent discoloration of the skin.
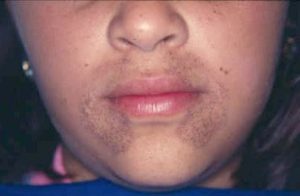
Figure 1: Discoloration from long-term usage of hydroquinone-containing products. Source: Wikimedia
Mercury, another dominating ingredient in many unregulated SLPs, inhibits skin pigmentation production to reveal a lighter complexion. The well-documented report from the Pan American Health Organization list of associated risks from mercury poisoning does not look so pretty.
Risks from prolonged exposure to mercury ranges from multiple major organ failures to psychological issues such as psychosis, depression, anxiety, and early-childhood development issues.
Minnesota Department of Health revealed that most SLPs contain mercury ranging from 135 to 33,000 parts per million (ppm). This level is much higher than the recommended level of 1 ppm by the US Food and Drug Administration (FDA).
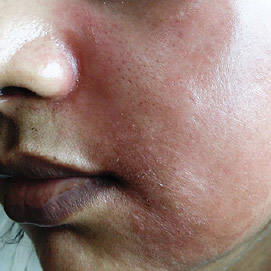
Additionally, the high levels of corticosteroids in most SLPs have resulted in steroid-dependent reactions (SDRs) – the tell-tale signs of chronic misuse of corticosteroids over an extended period (FIgure 2). Rashes, skin sensitivity to sunlight, and infections occur when SDR patients withdraw from topical steroids.
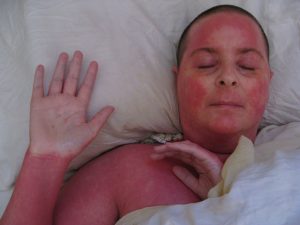
Figure 2: Steroid-Dependent Reactions. Source: Wikimedia
While these active ingredients in SLPs are effective in brightening consumers’ skin complexions along with providing additional skin care benefits, they pose significant and unwarranted health risks. Personally, the turmoil from experiencing side effects of some over-promising SLPs that are constantly promoted on social media is not worth sacrificing the highly-appraised fair skin. Not to mention the society that pressures those who are chasing after a fairer skin might be the first to criticize the consumers if SLP’s side effects arise. It is therefore crucial to pressure the SLPs’ manufacturers to move away from these dangerous compounds and towards safer ingredients.