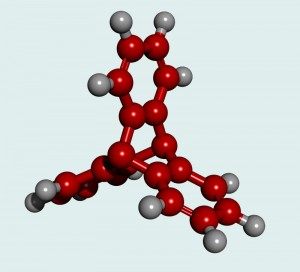
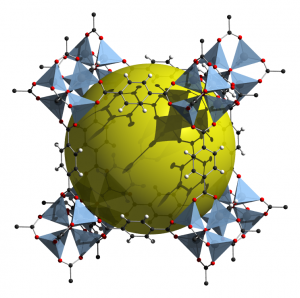
Hydrogen is considered as one of the most volatile elements known to man. Yet, if this explosive hydrogen gas can be safely stored it can instead be used as a new fuel source, which would benefit the world at large. Recent advances in chemical engineering have produced a family of materials, with the ability to efficiently adsorb (store) hydrogen gas. These materials, known as Metal-organic frameworks (MOFs), are a molecular structure that allows us the opportunity to take advantage of hydrogen as a fuel source. There are several benefits to using hydrogen as an energy source, one being that it can be readily produced for domestic use. For example, hydrogen can be generated from natural gas and biogas sources, as well as through the electrolysis (splitting) of water. This is favourable because the sources required to produce hydrogen are renewable, thus there is no need to worry about production shortages. Another advantage is that hydrogen is eco-friendly, in that using it in a fuel cell does not produce any greenhouse gases or air pollutants, and is not contributing to the effects of global warming.
In the following video, the basis of metal-organic frameworks in hydrogen gas storage is discussed, along with the associate research published by UBC graduate student Angela Crane:

With so many potential benefits, you may wonder why MOF technology is not currently being utilized in hydrogen-fueled vehicles, to provide a viable green alternative. The problem lies with the MOF’s mechanism of hydrogen adsorption and desorption (release), where the flow of hydrogen in and out of the structure is, for the most part, only through methods involving extreme cooling and heating. This system of temperature-regulated gas delivery enables precise control over hydrogen flow and greater storage capacities, however it is an impractical system to adopt in vehicles.
To remove the need of temperature for driving gas regulation and improving overall storage in the MOF structure, scientists are actively searching to optimize the material. They do this by increasing the available hydrogen binding sites and encouraging optimal pore-size, meaning hydrogen is better able to enter and remain in the MOF. In her research, Angela Crane investigats triptycene and pentiptycene, two large organic linkers with the potential for optimal pore size and orientation, thus being favourable in adsorbing hydrogen. When the MOF’s were tested for gas adsorption, however, she discovered that the complexity of the structure led to blockage of the pore-openings. This finding illustrates how the mechanics behind metal-organic frameworks are more complex than what one would expect from its relatively simple molecular structure.
Hydrogen fuel cells have the potential to revolutionize how we power the world. These devices lack all the liabilities associated with more conventional fossil fuels; most importantly pollution, and even other, less recognized concerns such as global conflict and of the depletion natural resources. The current state of hydrogen fuel cell research has some significant drawbacks that will have to be addressed in order for this technology to become a viable alternative energy source. Currently, conventional methods for producing hydrogen gas relies on the use of methane, a fossil fuel, making this process inherently unsustainable. There is hope, however, in the way of MOFs, which may one day provide an effective storage medium for hydrogen gas, if a sustainable method of hydrogen synthesis can be found.
Information on the drawbacks of hydrogen fuel is available below:
Audio clip: Adobe Flash Player (version 9 or above) is required to play this audio clip. Download the latest version here. You also need to have JavaScript enabled in your browser.
While the focus of metal-organic frameworks has been primarily based on hydrogen storage, MOFs have shown potential in various other applications as well. MOFs are currently being implemented for a variety of uses; acting as filtration systems, drug delivery components, fluorescent-imaging vectors and catalytic systems, to name a few. Due to the relatively simple production process, MOFs have now become commercially mass-produced. With their basic structure and efficient manufacture, the future for metal-organic frameworks is not limited to hydrogen gas storage, and its broad spectrum of use gives these frameworks unprecedented potential.
-Natasha Smyrnis, Sungbin Choi, Gurneet Kalra
Group2
References:
The New Chemistry of MOFs, Metal Organic Frameworks