An overview
Phage display is a very strong technique in drug discovery and development. It has many applications in improving the immunological studies such as immunotherapy, diagnosis, and development of monoclonal antibodies and peptides. The technique was initially demonstrated by George P. Smith in 1985. Its use for the production of recombinant antibodies was confirmed by McCafferty and his colleagues in 1990. Since then, a number of research groups have represented essential improvements of the technique for its applications in biological sciences. This technique allows for expression of a protein molecule such as an antibody on the surface of a bacteriophage.
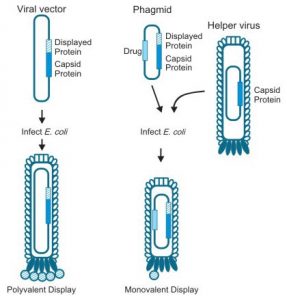
Phage display system. Source:https://www.cusabio.com/Phage-display-service.html
Phage expression system
Phage show differs from traditional expression systems in that the foreign gene sequence is spliced into one of the phage coat proteins, so that the foreign amino acid sequence is genetically fused to the amino acids of the coat protein to make a hybrid fusion protein. The hybrid coat protein is included into phage particles or virions as they are released from the cell, so that the foreign peptide or protein area is displayed on the outer surface.The foreign peptide might be fragments of antibody molecules containing fragment antigen binding (Fab) or single chain fragment variable (scFv). Both Fab and scFv are parts of antibody, which bind to antigen and expression of these parts on bacteriophage could be very helpful for study of antigen antibody interactions.
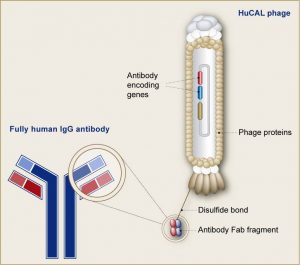
Phage_basic-WebGrafiken. Displaying of Fab fragment on bacteriophage. Source:https://www.morphosys.com/science/drug-development-capabilities/hucal
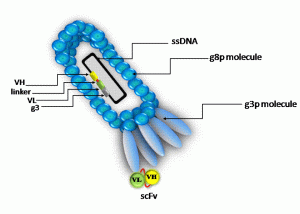
Displaying of scFv on bacteriophage. Source:http://www.kck.usm.my/diagnosticbook/?q=content/chapter-24-generation-recombinant-antibodies-display-technologies-diagnostic-applications
Phage display provides selection of specific antibodies
The produced phage antibodies are very important tools. It is possible to select specific antibodies against a desired antigen among phage antibodies, which received different antibody genes in an antibody library. The selected specific antibodies facilitate both immunotherapy and diagnosis.
The following video demonstrates the phage display system and selection of specific antibody in an antibody library.
Video taken from: https://www.youtube.com/watch?v=AqQDZxoCGqE
Application of phage display
Phage antibodies have been useful for immunization therapies, which may lead to development of new tools used for treatment of cancer, autoimmune and neurodegenerative disease. The utilization of some phage antibodies against breast, colorectal and pancreatic cancer shown some promising results regarding the cell growth inhibition of the cancer cells. The usage of phage display antibodies in designing diagnostic and therapeutic agents for autoimmune disease is growing. In Myasthenia gravis Fab antibodies against nicotinic acetylcholine receptors (AChR) caused reduced function of receptors at the neuromuscular junction. The role of selected phage antibodies in neurological disorders are also reported. Intracellular antibody fragments are potential therapeutic agents for neurological disorders due to their specificity for recognition of unusual intracellular proteins. In addition, the importance of phage display in hematological applications is shown. Anti-ABO, anti-Rh and anti-Kell antibodies were the first antibodies selected against red blood cells for hemagglutination assays.
Phage displayed peptides seem to be better molecular imaging agents compared to radiolabeled antibodies due to their small size, rapid blood clearance and tissue penetration. Recently, the application of phage display technique in the vaccine development and delivery is also described. The prominent role of phage display in healthcare, medicine and diagnostic will continue to expand.
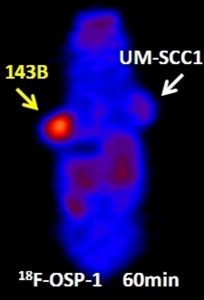
Phage Display Derived Peptides for Osteosarcoma Imaging. Source:http://www.wmis.org/abstracts/2010/forSystemUse/papers/P0844B.html
– Setareh Moazen
