Neuroimmune Signaling
There are two primary cell types in the CNS: neurons, responsible for transmitting electrical and chemical signals, and glial cells, which maintain homeostasis in the brain environment. Microglia and astrocytes are the main glial cell types in the CNS. Microglia – the brain-resident macrophages – support neurodevelopment by secreting neurotrophic factors and clearing cellular debris, protein aggregates, and dying cells (reviewed by Wiens et al., 2024, for references see Lab Publications page). Additionally, microglia can adopt various reactive phenotypes in response to endogenous or exogenous stimuli and elicit neuroimmune responses by secreting cytotoxic compounds such as nitric oxide (NO), reactive oxygen species (ROS), and pro-inflammatory cytokines (reviewed by Lindhout et al., 2021). While acute activation of microglia is required for immune defense, these cells are known to sustain chronic reactive states in many neurodegenerative conditions. Microglia in diseased brains release cytotoxic levels of pro-inflammatory mediators into the extracellular space, resulting in neuronal death, cognitive impairment, and memory deficits – hallmark features of AD. Astrocytes play a multifaceted role in maintaining neuronal health by upkeeping the blood-brain barrier, assisting with synapse formation and elimination, engaging in phagocytic activity, and contributing to inflammatory responses. Maintaining homeostasis in the brain involves complex communication mechanisms between neuronal and non-neuronal cells, and the precise roles of microglia and astrocytes in modulating neuroinflammation remain poorly understood. For example, we are only beginning to appreciate the full complexity of microglia to astrocyte signaling (reviewed by Mohammad et al., 2024). Therefore, research towards better understanding the pathophysiology of disease-associated microglia and astrocytes is fundamental for identifying effective therapeutic strategies and slowing the progression of neurodegenerative disease.
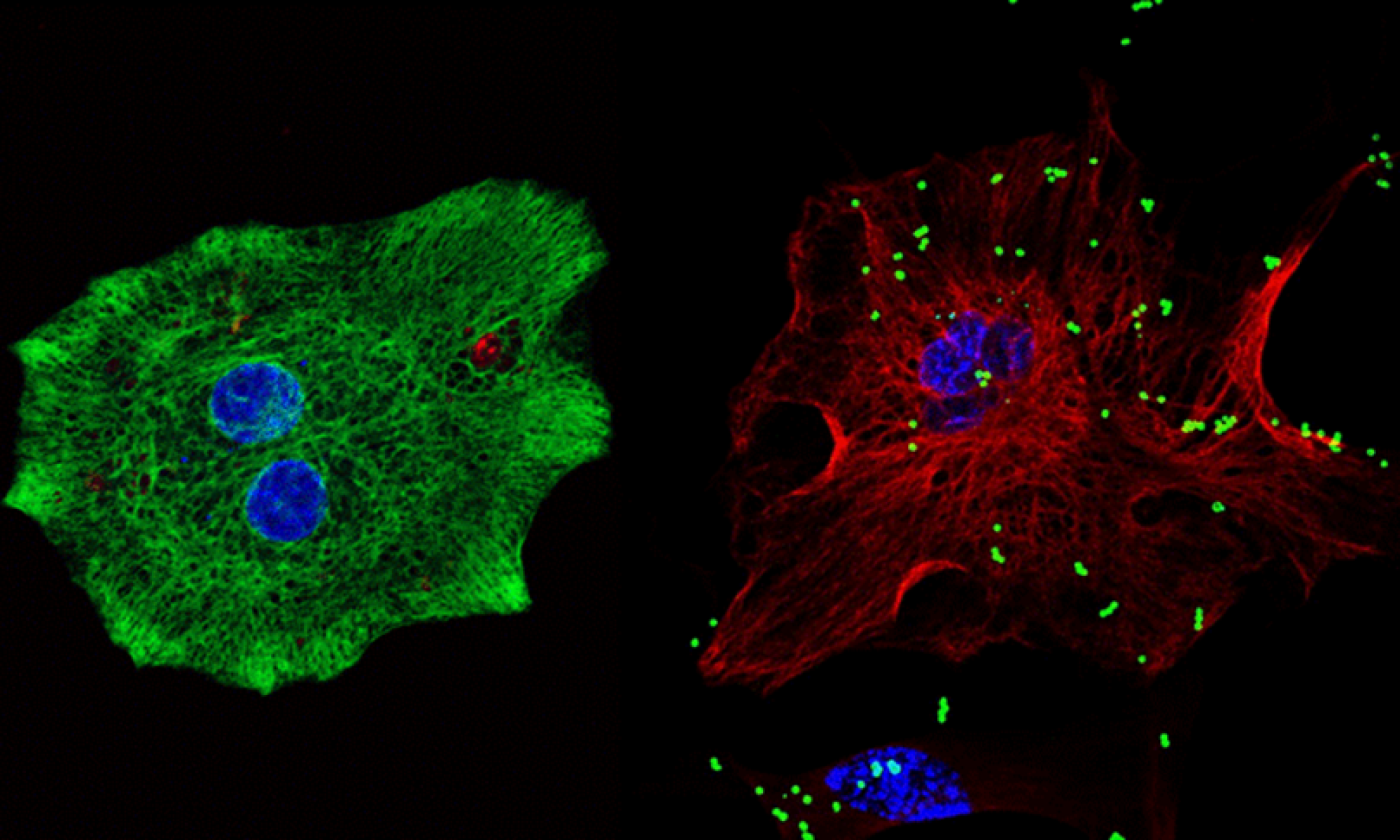
bisbenzimide + fluoresbrite + GFAP.
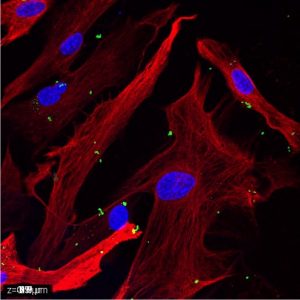
Microglia and astrocytes are both targets and sources of signaling molecules, such as chemokines, cytokines, and growth factors, that are essential for crosstalk between different CNS cell types. To this end, we have extensively studied the roles of various signaling molecules in neuroimmunomodulation by these cell types. Our findings reveal distinct responses by murine microglia to a variety of immune stimulants, including exogenous lipopolysaccharide (LPS), polyinosinic:polycytidylic acid (poly I:C), and zymosan A, and endogenous interferon (IFN)-ß and IFN-γ (Bernath et al., 2023). Additionally, we have characterized LPS, IFN-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-1ß, and extracellular cytochrome c (CytC) and adenosine triphosphate (ATP) as key regulators of human and murine astrocyte phagocytic activity (Yang et al., 2023, 2025). These results demonstrate the dependence of neuroimmune responses on the microenvironment of cells and highlight existing differences between glial cells derived from different species.
Damage-associated molecular patterns (DAMPs)
In addition to the known exogenous triggers of CNS neuroinflammation, our lab has identified additional key players in the molecular mechanisms of neurodegeneration in the form of damage-associated molecular patterns, or DAMPs. DAMPs are intracellular compounds that can be released into the extracellular space from damaged or dying cells where they act as signaling molecules to elicit an acute inflammatory response in immune cells. In disease conditions, the accumulation of dying neurons results in toxic levels of DAMPs in the extracellular milieu, exacerbating the inflammatory cycle (reviewed by Klegeris, 2020; Wenzel et al., 2020). There are several already well-described CNS DAMPs that under normal physiological conditions reside in the nucleus (e.g., high-mobility group box 1 or HMGB1 protein) or mitochondria (e.g., mitochondrial DNA, N-formyl peptides), but many more DAMP-like molecules have been characterized in peripheral tissues. One of the research goals of our lab has been the identification of novel DAMPs in the CNS, which is essential for understanding the pathogenesis of AD and other neurodegenerative disorders.
Histones are one such class of molecules that we have focused on. They are a family of highly conserved DNA binding proteins that play an essential role in epigenetic regulation. Histones are known to be released into the extracellular space by dying cells, where they act as DAMPs on peripheral immune cells, but there is very limited research on their actions in the CNS extracellular space (reviewed by Richards et al., 2022). Our lab has identified linker histone H1 and core histone H3 as modulators of neuroimmune responses in the CNS, which upregulate the production of NO, TNF-α, and IFN-γ-inducible protein (IP)-10, in human and murine microglia-like cells. Moreover, histone H1 downregulates phagocytic activity – an important homeostatic process – in murine microglia and is directly cytotoxic to both human and murine neuronal cells (McRae et al., 2024). A mixture of several histones exhibits more complex regulatory effects on microglia and astrocytes, and is directly toxic to neurons (Da Silva et al., 2024).
Cytochrome c (CytC) is a metalloprotein normally located in the mitochondrial intermembrane space, where it facilitates electron transport necessary for ATP production. When released into the cytosol by damaged mitochondria, CytC acts as a signaling molecule for the initiation of apoptosis. Recent evidence indicates that CytC can be released into the extracellular space by dying cells, suggesting potential DAMP-like properties (reviewed by Brooks, 2025; Klegeris, 2020; Bajwa, 2019). We established that CytC exhibits DAMP activity within the CNS, where, upon recognition by microglia, it elicits a pro-inflammatory response characterized by increased ROS and NO production, and enhanced cytotoxicity of immune-stimulated microglia-like cells (Gouveia et al., 2017). Furthermore, extracellularly applied CytC increases immune activation of primary human astrocytes by upregulating the secretion of IL-1β, IL-12 p70, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Wenzel et al., 2019). The mitochondrial phospholipid cardiolipin is another DAMP-like molecule our lab has recently studied (see Wenzel et al., 2023, 2021; Murray et al., 2022; Pointer et al., 2019). Interestingly, cardiolipin can exhibit both pro- and anti-inflammatory activity, which appears to depend on the cell type it interacts with.
Anti-inflammatory mediators
Our group has contributed to evidence supporting the hypothesis that neuronal death results from the aberrant activation of microglia, in part by studies demonstrating the neuroprotective mechanisms of nonsteroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory compounds. These investigations support the hypothesis that normalizing the immune responses of disease-associated microglia represents a viable target for slowing the progression of neurodegenerative diseases. To this end, our laboratory has identified different types of low-molecular-weight compounds capable of crossing the blood-brain barrier, that hold promise as therapeutic agents for reducing chronic neuroinflammation (Greuel et al., 2024; Alford et al., 2019; McKenzie et al., 2019). Additionally, psychedelics, such as psilocybin and lysergic acid diethylamide (LSD), exhibit certain anti-inflammatory properties and have been explored as potential treatments for neuroinflammatory pathologies, including depression. Recently, we have demonstrated that psilocin, the bioactive metabolite of the psychedelic alkaloid psilocybin – which was first isolated from mushrooms of the Psilocybe genus – regulates the immune functions of microglia in a way that may be beneficial for treating neurodegenerative disorders (Wiens et al., 2024). We continue to investigate new anti-inflammatory compounds with potential as inhibitors of chronic inflammation in the CNS.
Significance
Currently, no effective disease-modifying therapies are available for AD. Therefore, continued studies of the molecular mechanisms of this disorder are critical to gain a deeper understanding of disease progression and develop viable treatment strategies. If you are interested in contributing to this research, please contact Dr. Andis Klegeris at andis.klegeris@ubc.ca for more information.