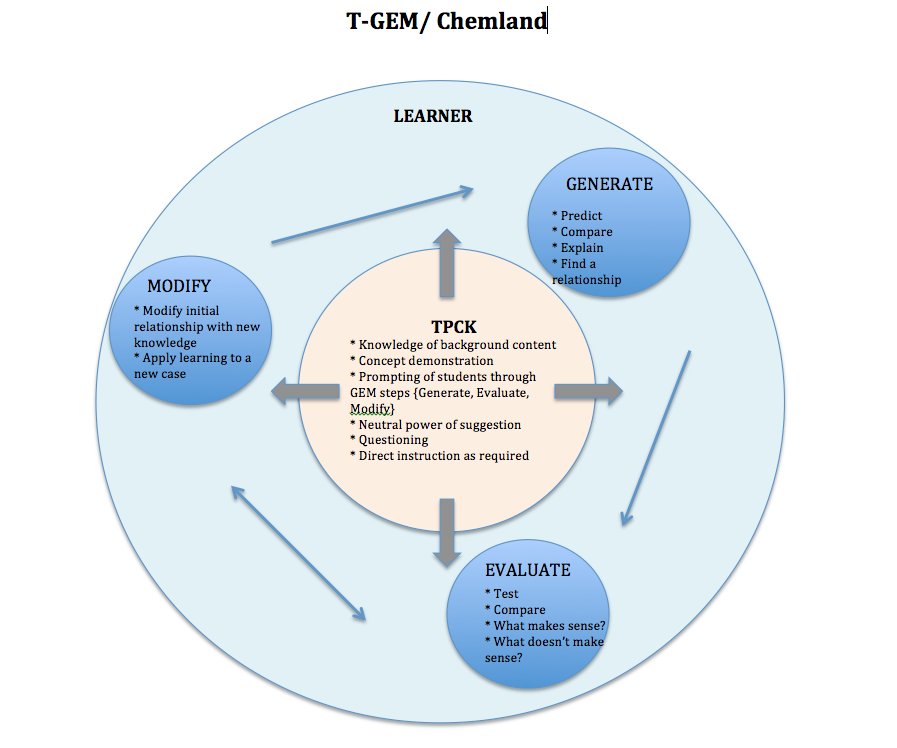
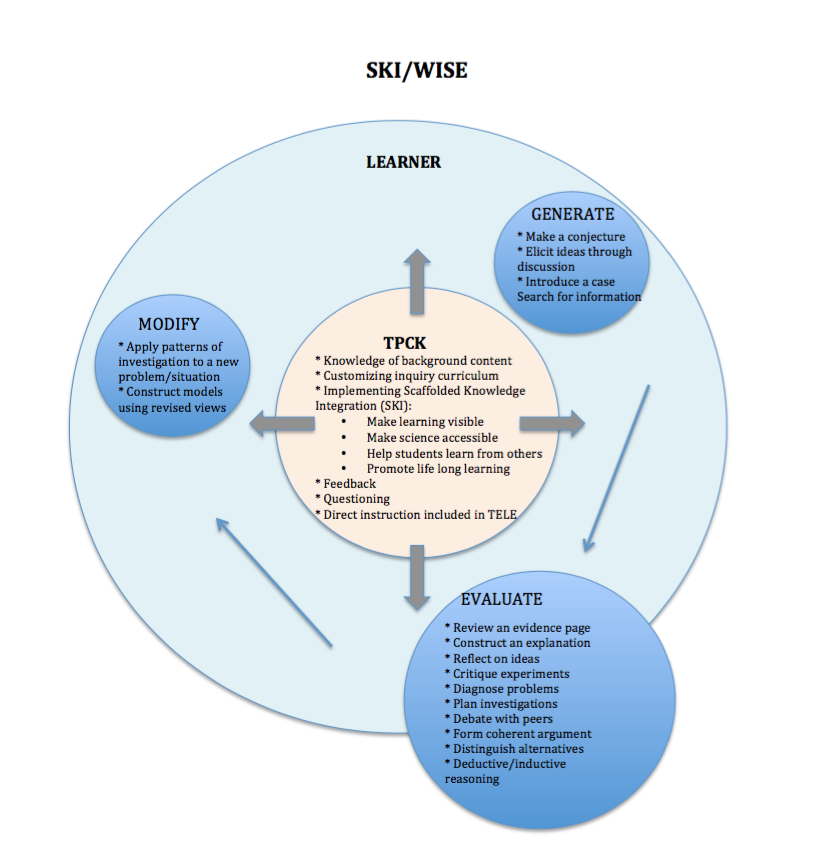
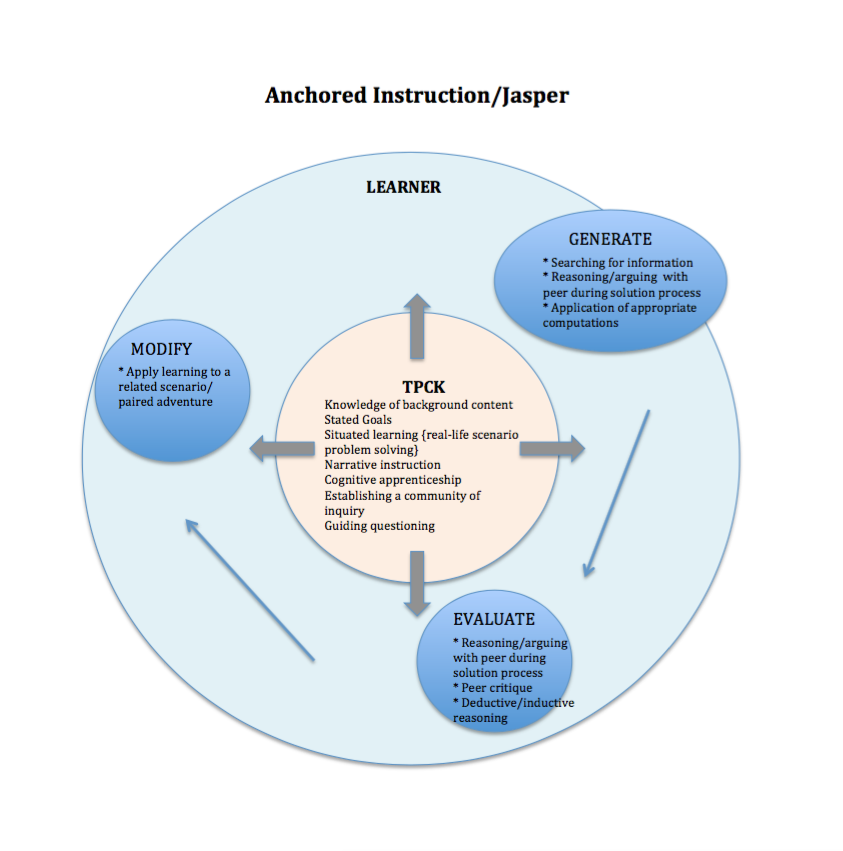
Following is a collection of visual syntheses comparing and contrasting T-GEM/Chemland with the following technology-enhanced learning environments: Learning for Use (LfU)/My World, Scaffolded Knowledge Integration (SKI)/WISE, and Anchored Instruction/Jasper. The visual syntheses contain a focus on TPCK and learner activity with the guiding TELE being T-GEM/Chemland, and all other TELEs being compared and contrasted through alignment with the T-GEM/Chemland framework.
Each one of these TELEs is developed on inquiry instruction and learning, with T-GEM/Chemland consisting of specifically model-based inquiry. Each one of these TELEs promotes a community of inquiry with purposeful teacher-student and student-student interactions. To emphasize the non-linear processes of inquiry, each visual synthesis is designed in a circular format.
Unique to T-GEM is the cyclical progress that the learner takes moving through the steps of the learning theory. Arrows are placed in each TELE’s visual representation to elicit the learner’s movement in comparison to the T-GEM’s model.
 As a general mathematics and science teacher for elementary grade levels, the process of exploring, analyzing and synthesizing the four foundational TELEs presented in this course has been transformational in my development of TPCK. Initially, the importance of CK (Schulman, 1986), and my self-diagnosed lack of CK, was convicting as I tend towards growing in pedagogical ideas and creative ways of implementing them. To further this conviction, my understanding of inquiry processes and the intricate role that the teacher facilitates in conducting a community of inquiry began to become clearer throughout the readings and discussions of Module B. Skillful inquiry instruction requires a facilitator who is saturated in CK, being equipped to prepare, respond, question, prompt, and guide with carefully considered PK. At this time, I am challenged as an educator to begin with one brave adventure in mathematics using an anchored instructional approach, and another brave lesson in physical science using a T-GEM approach. I am certain that I will be generating, evaluating and modifying all along the way.
As a general mathematics and science teacher for elementary grade levels, the process of exploring, analyzing and synthesizing the four foundational TELEs presented in this course has been transformational in my development of TPCK. Initially, the importance of CK (Schulman, 1986), and my self-diagnosed lack of CK, was convicting as I tend towards growing in pedagogical ideas and creative ways of implementing them. To further this conviction, my understanding of inquiry processes and the intricate role that the teacher facilitates in conducting a community of inquiry began to become clearer throughout the readings and discussions of Module B. Skillful inquiry instruction requires a facilitator who is saturated in CK, being equipped to prepare, respond, question, prompt, and guide with carefully considered PK. At this time, I am challenged as an educator to begin with one brave adventure in mathematics using an anchored instructional approach, and another brave lesson in physical science using a T-GEM approach. I am certain that I will be generating, evaluating and modifying all along the way.
Cognition and Technology Group at Vanderbilt (1992). The jasper experiment: An exploration of issues in learning and instructional design. Educational Technology Research and Development, (40), 1, pp.65-80
Edelson, D.C. (2001). Learning-for-use: A framework for the design of technology-supported inquiry activities. Journal of Research in Science Teaching,38(3), 355-385.
Khan, S. (2007). Model-based inquiries in chemistry. Science Education, 91(6), 877-905.
Linn, M. C., Clark, D. and Slotta, J. D. (2003), WISE design for knowledge integration . Sci. Ed., 87: 517–538. doi:10.1002/sce.10086
Shulman, L.S. (1986). Those who understand: Knowledge growth in teaching. Educational Researcher, 15(2), 4 -14.