The ones who are most susceptible to falling ill from Listeria monocytogenes are pregnant women and their unborn or newborn children, seniors and the immunocompromised. For pregnant women in the first three months of pregnancy, being sick with Listeria monocytogenes can cause a miscarriage. If the bacteria is contracted later on in the pregnancy, premature birth, stillbirth or the birth of a severely ill child may happen. The immunocompromised are much more likely to get sick but according to the Public Health Agency of Canada, people suffering from AIDS are 300 times more susceptible to being infected by Listeria monocytogenes compared to healthy individuals. (Public Health Agency of Canada, 2012)
Listeria Monocytogenes luckily cannot grow in all ready to eat food products as long as the food product falls under one of the following three criteria according to Australia’s Food Standards (Food Standards, 2014):
1. pH less than 4.4, no matter the water activity value
2. Water activity less than 0.92, no matter the pH value
3. pH less than 5.0 and water activity less than 0.94
If the ready to eat food product is frozen and is consumed frozen, thawed but still eaten cold or heated before consumption then it is most likely safe from Listeria Monocytogenes. (Food Standards, 2014) If the ready to eat food product does not fit with the above criteria, then heating to an internal temperature of 74°C before eating can help in minimizing the chance of Listeria monocytogenes surviving in the food. (Public Health Agency of Canada, 2012)
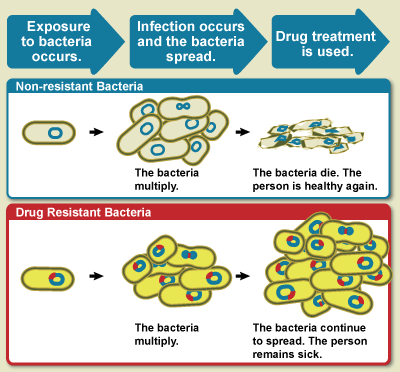
With all the conditions Listeria monocytogenes can grow or survive in ready to eat products, I feel that one of the better ways to minimize the risks of getting ill from Listeria monocytogenes is to heat ready to eat products except for frozen products before consuming. Although this might be difficult for ready to eat foods that are generally eaten at room temperature such as sandwiches.
Are there any other methods that you think are sufficient in eliminating Listeria monocytogenes?
References:
Andersen, L. (2015) Listeria and Bacteriocin-Producing Starter Culture. Retrieved from http://www.foodsafetynews.com/2015/08/listeria-and-bacteriocin-producing-starter-cultures/#.Vi3XUmSrToB
Australian Competition Consumer Commission. (2015). Product Safety Recalls Australia. Retrieved from http://www.recalls.gov.au/content/index.phtml/itemId/1076441
Food Standards. (2014). Supporting document 1 – Guidance On the Application of Microbiological Criteria for Listeria Monocytogenes. Retrieved from
http://www.foodstandards.gov.au/code/proposals/Documents/P1017-MicroAppR-SD1.pdf
Lawley, R. (2013). Food Safety Watch. Retrieved from http://www.foodsafetywatch.org/factsheets/listeria/
Public Health Agency of Canada. (2012). Listeria. Retrieved from
http://www.phac-aspc.gc.ca/fs-sa/fs-fi/listerios-eng.php








 half of the infected people are children younger than 18 years old.
half of the infected people are children younger than 18 years old. 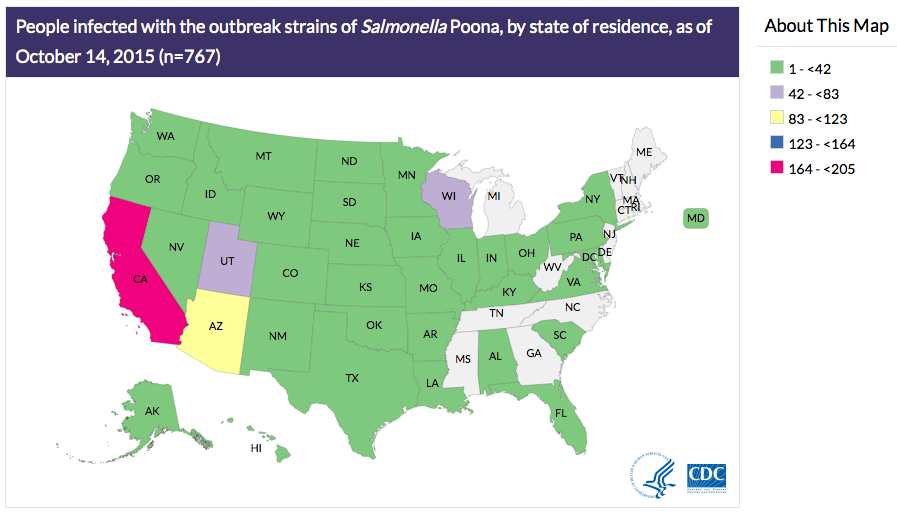



 Numerous studies have specifically examined the survival and growth of E. coli on leafy vegetables. For instance, Parker et al. (2011) demonstrated E. coli’s “ability to multiply in the phyllosphere of whole lettuce plants” on shredded and intact harvested lettuce leaves, due to an up-regulation of genes involved in oxidative and osmotic stress, which also make the bacteria more resistant to antimicrobials commonly used in the fresh-cut produce industry. Therefore, the food industry needs to implement more effective strategies in handling raw vegetables.
Numerous studies have specifically examined the survival and growth of E. coli on leafy vegetables. For instance, Parker et al. (2011) demonstrated E. coli’s “ability to multiply in the phyllosphere of whole lettuce plants” on shredded and intact harvested lettuce leaves, due to an up-regulation of genes involved in oxidative and osmotic stress, which also make the bacteria more resistant to antimicrobials commonly used in the fresh-cut produce industry. Therefore, the food industry needs to implement more effective strategies in handling raw vegetables.