Xiaoqi Li | MEL Candidate | Dec, 2024.
Mentor: Jeffrey Vanderkley, P.Eng. , FortisBC
Introduction
Catalyst Methane Pyrolysis (CMP) is a highly endothermal reaction requiring a heat input of 74.85 kJ/mol at 298 K.
CH4 –> 2H2 + C
The Hazer Process uses iron ore as a catalyst to facilitate methane decomposition, producing hydrogen and solid graphite. This dual-output process aligns with clean energy goals for diverse industrial applications of graphite [1].
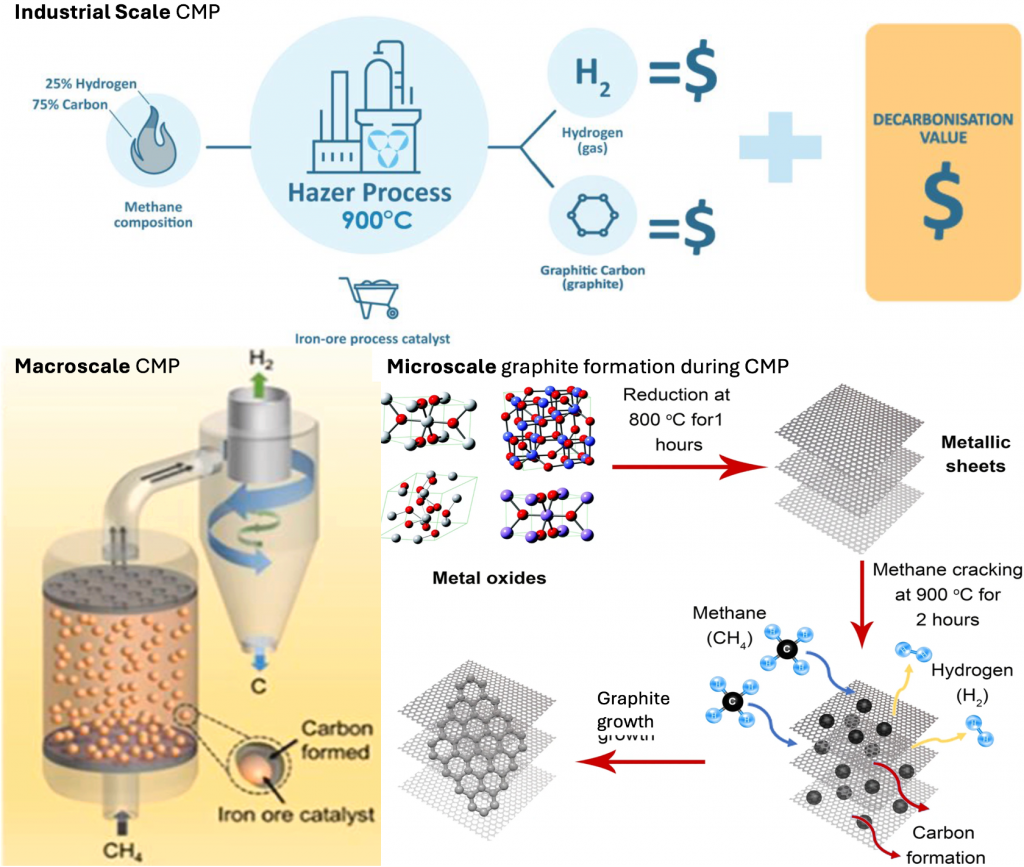
Figure 1. From industrial scale to micro-scale catalyst methane pyrolysis process [3,5].
Graphite Properties
(1) Unique structure of graphite, consisting of stacked layers of hexagonally arranged carbon atoms bonded by strong covalent bonds within the layers. This arrangement allows electrons to move freely within the planes, giving it excellent electrical and thermal conductivity. (2) Weak van der Waals forces enable the layers to slide over each other, resulting in its lubricity and softness. (3) The high degree of crystallinity and structural stability contribute to its thermal resistance and inertness.

Figure 2. Graphite properties words cloud.
Graphite of End-Use Applications
Purity of graphite is a critical measure that determines its structure and suitability for various applications, which depends heavily on the operational conditions of methane pyrolysis. Factors such as catalyst type, temperature, methane concentration, gas flow rate, reactor design, and heating methods play a significant role in defining the purity, morphology, and crystallinity of the graphite produced.
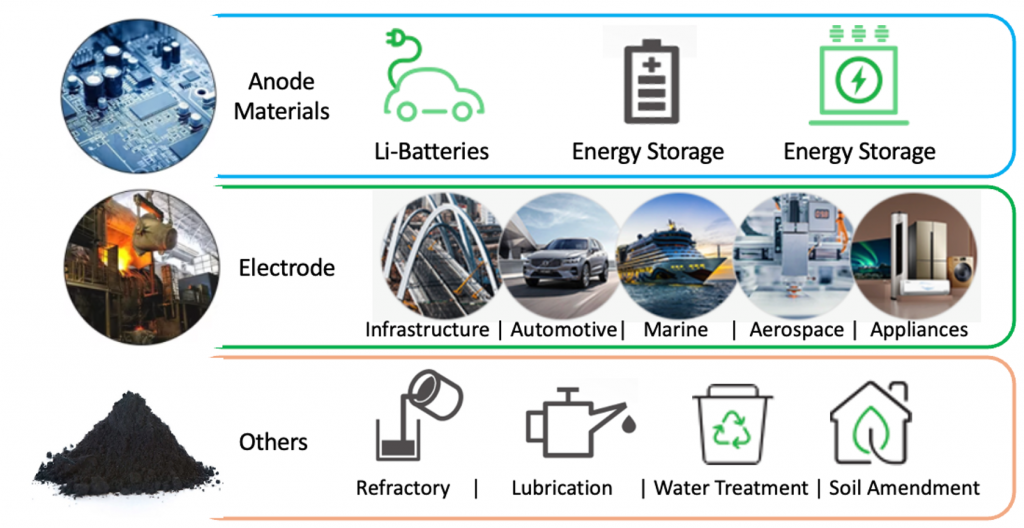
Figure 3. Graphite applications and end-use industries[2].
Graphite Market
Graphite market size has grown strongly to $1.3 billion in 2024. It is expected to see strong growth at a compound annual growth rate (CAGR) of 7.4% in 2028. This trends can be attributed to demand from steel industry, battery and energy storage.

Figure 4. Synthetic graphite demand in industries (right)[3] and raphite market size (left)[4]
Conclusions
Catalytic methane pyrolysis, exemplified by the Hazer Process, leverages iron ore catalysts to produce hydrogen alongside high-value synthetic graphite. This graphite, with its diverse industrial applications in batteries, steelmaking, and advanced manufacturing, represents a critical byproduct with significant economic and technological potential. The process offers a sustainable pathway for low-carbon hydrogen production while positioning graphite as a key material for advancing clean energy solutions and driving decarbonization efforts.
References
[1] Pocock, G., Cornejo, A., & Chua, H. T. (2016). A process for producing hydrogen and graphitic carbon from hydrocarbons
(World Intellectual Property Organization Patent No. WO2016154666A1).
[2] Hazer Group Limited. (2024). Canadian Project Update.
[3] ECGA. Main uses of carbon and graphite. https://ecga.net/main-uses-of-graphite/
[4] Graphite Market Report 2024—Graphite Industry Research and Market Report. November 25, 2024.
[5] Prabowo, J., Lai, L., Chivers, B., Burke, D., Dinh, A. H., Ye, L., Wang, Y., Wang, Y., Wei, L., & Chen, Y. (2024). Solid carbon
co-products from hydrogen production by methane pyrolysis: Current understandings and recent progress. Carbon, 216,
118507. https://doi.org/10.1016/j.carbon.2023.118507
Contact
Hazel Li
hazel1016li@gmail.com