“mono-alkyl esters of long chain fatty acids derived from vegetable oils and animal fats” … What?
Let’s break it down together. Time to learn some organic chemistry! Organic chemistry is the study of carbon compounds and involves a lot of shorthand. Let’s start at the beginning.
Mono-alkyl
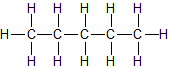
This means an un-branched chain of carbon atoms (C), where each C has has four single-bonds. Hydrogen atoms (H) fill the gaps. These chains of carbon atoms are normally known as “alkanes”, but when an alkane is attached to something else, the “-ane” ending is replaced with “-yl”.
We’ll spare you the long explanation how chemical bonds work, but you should know that chemical bonds store the energy that is released when fuel is burned.
Esters
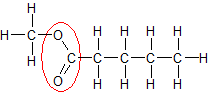
An ester is an organic chemistry functional group, defined by a carbon atom (C) double-bonded to an oxygen atom (O) and single-bonded both to a C and a second O, which is in turn bonded to another C. In the diagram, the ester functional group is circled in red.
The two C at either end of an ester can connect to other Cs, like the chain in the example diagram does.
Long Chain Fatty Acids
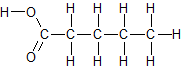
“Fatty acid” means a carboxylic acid. A carboxylic acid is like an ester, except there is only an H instead of a C next to the O. That H is acidic, meaning it is easily separated fom the rest of the molecule. Esters are often made from carboxylic acids, are are easily “saponified” back to conjugate bases of carboxylic acids. You’ll see more about saponification if you read about difficulties in biodiesel production.
“Long chain” means that the chain of Cs in your alkyl group is long. In organic chemistry, that usually means a ballpark of at least 10 carbon atoms.
Vegetable Oils and Animal Fats, AKA Triglycerides
How do these all fit together? The trans-esterification reaction.

