We study the somatic genome of metastatic genitourinary cancers, using custom experimental and bioinformatic platforms developed in our laboratory. Each year, we sequence thousands of cell-free DNA and tumour tissue DNA samples, across a range of different clinical scenarios, and with a focus on samples collected in the framework of phase II and III clinical trials, where clinical outcomes data are robust. There are a wide range of hypotheses that we are testing, but here are a few ongoing projects that give insight into our overall research program:
Predicting response and resistance to systemic therapy in metastatic prostate cancer
Recent years have seen the approval of multiple systemic therapies targeting persistent AR signalling in metastatic castration-resistant prostate cancer (CRPC). However, many patients do not initially respond and all patients eventually progress. There are also unanswered questions as to optimal treatment selection. The major challenge in addressing these issues is the absence of treatment-predictive biomarkers for CRPC patients.
Through close collaboration with Dr. Kim Chi, we have access to thousands of plasma cell-free DNA samples from Canadian CRPC patients receiving systemic therapy. A large proportion of these samples have accrued via phase II clinical trials. We apply genomics and bioinformatics approaches to characterize the circulating tumour DNA (ctDNA) present in these ‘liquid biopsies’. We aim to better understand the link between tumour molecular subtype and patient response to therapy. Ultimately, we hope to develop a clinically-practical framework (requiring only a blood sample) that could prioritize patients for therapies with the best chance of success.
Development of liquid biopsy strategies for metastatic bladder cancer
Metastatic bladder cancer is a lethal and under-studied disease. Chemotherapy has historically been the most effective treatment for metastatic disease, but long-term survival has been a rarity. Recently, new drugs including immunotherapies and targeted therapies are showing significant promise, but only a small proportion of patients respond to these treatments. We are currently testing the extent to which ctDNA from metastatic bladder cancer patients is concordant with matched tissue biopsy from the same patients.
Our work suggests that mutations detected in ctDNA accurately reflect cancer itself. We have identified alterations in the ctDNA of hundreds of Canadians with metastatic bladder cancer that are being treated with chemotherapy or immunotherapy. Currently, we are working to determine which mutations can predict a patient’s subsequent response to treatment using our rare combination of cancer bioinformatics and clinical oncology. Success with this work will also help explain why some metastatic bladder cancers are intrinsically responsive or resistant to the most commonly used therapies.
Detecting druggable DNA repair defects in metastatic prostate cancer
Global sequencing efforts have revealed that germline or somatic defects to DNA damage repair genes (e.g. BRCA2) are present in 20-30% of mCRPC patients and that these patients may be susceptible to genotoxic chemotherapies, DNA repair inhibitors, or even immunotherapy. There is an urgent need for practical biomarkers to identify patients with actionable DNA repair defects in their tumours, and for clinical development of effective therapeutic agents to exploit this cancer-specific weakness.
We are determining the precise frequency of therapeutically-targetable DNA damage repair defects that can be identified via liquid biopsy. We are interrogating the proportion of defects that are biallelic (i.e. lead to complete loss of wildtype function), and whether they are always associated with downstream signatures of defective DNA repair.
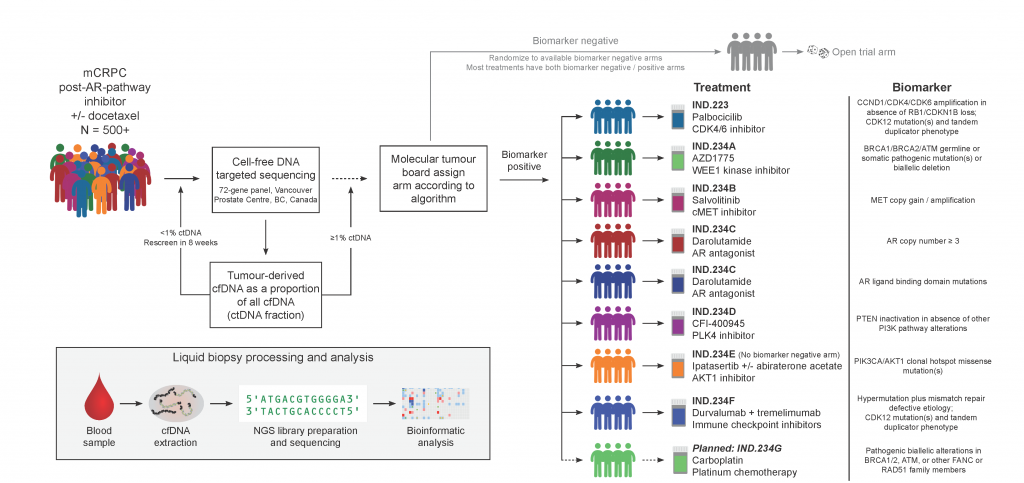
Testing ctDNA as a predictive biomarker in a precision oncology clinical trial
We direct the ctDNA screening strategy and the molecular tumour board for IND.223/232/234, a Canadian multi-center phase 2 umbrella trial in metastatic castration-resistant prostate cancer patients (NCT03385655, NCT02905318). In the trials, chaired by Dr. Kim Chi via the CCTG, patients are matched to various different targeted therapies on the basis of alterations found in pre-treatment circulating cell-free tumour DNA. By 2021, 435 Canadian patients have been screened as part of these trials.