Think about one chemical used in daily life. I guess most non-chemists would say water. Water is the basic necessity of all living organisms on Earth. It has a very simple structure: two hydrogen atoms stick with one oxygen atom, but it took the Universe 1.6 billion years to make the first water molecule and 13.6 billion years to make water on Earth!
Have you ever wondered why water is so vital to life? One of the answers is proteins. Proteins are the MVP in the body; they do almost everything in cells and make sure tissues to grow and organs to function. Proteins can fold into complex 3D structures, and their biological reactivity depends on how they fold. Although the mechanism remains unclear, studies indicate that interaction between protein and surrounding water controls protein folding. For example, using a theoretical protein-solvent model and a statistical physics approach, Oliver Collet from Nancy University in France suggests that the hydrogen bonding formed between water and proteins promotes fast protein folding as it is relatively easy to break and reform hydrogen bonds at a high temperature.
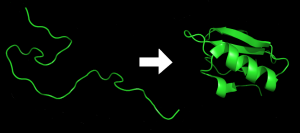
Protein before and after folding. Image taken from Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/a/a9/Protein_folding.png
In the Catcher in the Rye, the protagonist, Holden Caulfield, wonders what happens to the ducks and fish in Central Park when the large pond freezes. I don’t know about ducks, but I can tell Holden that fish stay at the bottom of the pond and survive over the winter because of the unique thermal properties of water. Hydrogen bonding pushes molecules further apart when water is frozen, so ice is less dense and floats. Oddly, at 4℃, liquid water expands on heating or cooling and has the highest density. This means that water at the bottom is the warmest and maintains a temperature around 4, allowing fish and other aquatic life to survive. In some extreme low-temperature environment, fish use supercooling to avoid freezing. Supercooling is when water is cooled below -40℃ without freezing, and you can even try at home.
How to make supercooled water at home. Video taken from YouTube
Although water is largely used as a solvent in most reactions, it has been found that water can act as an electron donor for certain biocatalytic reactions, enabling more efficient and greener synthetical routes. Avelino Corma, Frank Hollmann and co-workers report water as the electron donor for biocatalytic redox reactions using enzymes, like oxidoreductases. Despite the requirement of additional energy, activation of water as an electron donor is a common natural process: photosynthesis where visible light provides energy to promote water oxidation, generating oxygen along with electrons and protons. Inspired by nature’s method, the researchers come up with a strategy to accelerate water oxidation using photocatalysts, in this case, metal-doped TiO2.
As water is so common in our daily life, it is often underappreciated. It facilitates not only biological activities but chemical reactions and ensures all creatures to survive even under the harshest conditions on Earth
Reference:
Francl, M., Nature Chemistry, 2016, 8, 897-898
Collet, O., J. Chem. Phys, 2011, 132, 085107
Chaplin, M., Water structure and Science, http://www1.lsbu.ac.uk/water/water_anomalies.html#j (accessed Jan. 20, 19)
Supercooling, Wikipedia.org, https://en.wikipedia.org/wiki/Supercooling (access Jan. 20, 19)
Mifsud, M.; Gargiulo, S.; Iborra, S.; Arends, I.W.C.E.; Hollmann, F.; Corma, A., Nature Communications 2014, 5, 3145
One response to “The Awesomeness of Water”