Global temperature averages are increasing at abnormal rates. Sea levels are rising, ice caps are melting, and nature is dying. Almost all governments and scientists worldwide have been looking for solutions to impede the fate that humanity is heading towards. One of the ways is through using a cleaner energy source such as hydroelectricity.
In the past two decades, hydroelectricity has become more popular as a substitution for fossil fuel powered energy production. Its claim to fame was that it was a clean way to generate electricity; it was a viable solution for the future as climate change started to become a mainstay in the news. Initial studies had hydroelectricity being almost emission free!
Vattenfall Study for Carbon Emissions of Fossil Fuels, Renewables, Nuclear. Source: Wikimedia Commons
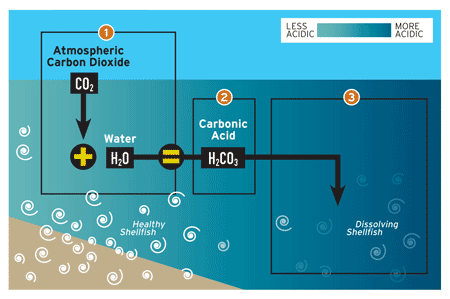
However, hydroelectricity was also declared a part of the greenhouse gas problem.
When thinking about producing electricity using water, one would think of it as being a clean and renewable process. It’s water! People bathe in it and drink it. How can it be dirty?
Unlike coal, a non-renewable resource which takes millions of years to form, the water isn’t being burned or used up. However, like coal , it is not clean (albeit to a much lesser extent).

Clean water for drinking. Source: kisspng
The basics of hydro-power is that water is pumped through a turbine to induce spinning. This motion activates a generator, producing the electricity which has become so important in daily life. The dirty part comes from the carbon emissions generated during construction and passively during the hydroelectric dam’s lifetime.
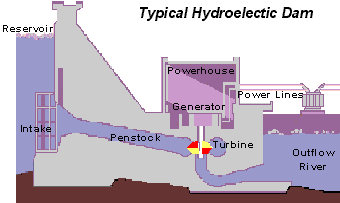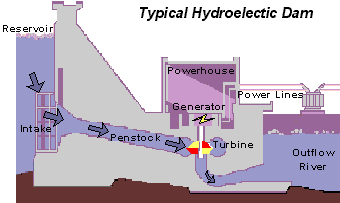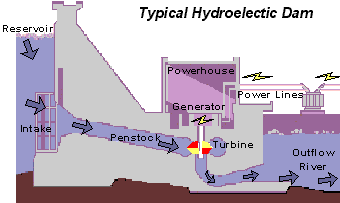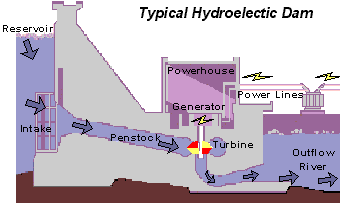
Typical hydroelectric dam. Source: United States Geological Survey (USGS)
Throughout the building process, many carbon sinks will be destroyed as trees and plants will be chopped down. This reduces the overall carbon dioxide that can be stored while releasing the carbon dioxide back into the atmosphere. Not to mention the habitats and the environments near the power plants that are destroyed at the same time.

Hydroelectric dam construction by China. Source: Giorgio Taraschi / Al Jazeera
However, it’s not just during the initial construction that hydroelectric power is unclean. Plants and other living things are able to grow in these bodies of water. All living things that die and decompose in the waters produce methane, carbon dioxide, and nutrients. The nutrients in turn help other living organisms grow, thus continuing the cycle. When thought about in this manner, the hydroelectric dams become carbon emission generators.
In its current state, hydroelectricity is not a clean source of power. Nevertheless, it is a viable alternative to fossil fuels as we transition to better solutions. There is hope in the future that hydro-power becomes one of these solutions. Companies are still innovating and working towards a setup that is gentler on the environment while still providing adequate amounts of electricity.