After World War II, thousands of synthetic chemicals became commercially available for use in agriculture, manufacturing, or disease control. Some of these chemicals were classified as “persistent organic pollutants”, or POPs, because of how they resist degradation, persist in the environment, and are toxic to plants or animals.
One well-known example is DDT, an insecticide that became infamous for its health and environmental impacts after Rachel Carson’s Silent Spring was published in 1962, and was ultimately banned for American agricultural use in 1972. Like many POPs, DDT magnifies along the food chain and accumulates in fish. In 2018, University of Maine researchers found that children who eat fish from rivers fed by the Eastern Alaska Mountain Range have a cancer risk above the Environmental Protection Agency’s threshold limit.
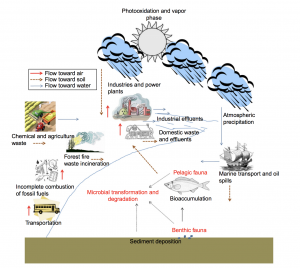
A biogeochemical cycle of PAHs in the environment. Source: Microbial Biodegradation and Bioremediation (Das et al. 2014)
In order to better understand the atmospheric chemistry of POPs, Colin Pike-Thackray—a graduate student in Dr. Noelle Selin’s group at the Massachusetts Institute of Technology—used quantitative models based on uncertainty. One class of pollutants that Pike-Thackray focused on in his thesis were polycyclic aromatic hydrocarbons (PAHs), which result from fuel or biomass consumption. Similar work in the field had revealed that uncertainty in simulations of DDT concentrations result from estimated emission and degradation constants, while uncertainty in simulations of mercury concentrations in the air and ocean surface were due to partition coefficients and reaction rate constants.
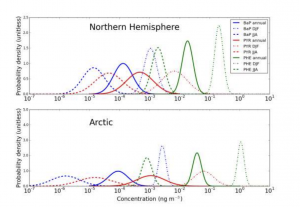
Uncertainty distributions for the atmospheric concentrations of different PAHs (colour-coded) in the Northern Hemisphere and the Arctic. Anuual (solid), winter averaged (dot-dashed), and summer (dotted) averages are shown. Source: Environmental Science and Technology (Pike-Thackray et al. 2015)
Using the mathematical method of “polynomial chaos”, in which each parameter of a dynamic system is a source of uncertainty, Pike-Thackray et al. (2015) found that a variety of factors increased the uncertainty of estimated PAH concentrations. One leading contributor was the black carbon-air partition coefficient, which describes the relative concentrations of PAHs trapped in in air or black carbon at equilibrium. The oxidation rate constants of PAHs were also significant sources of uncertainty. In addition to uncertainty arising from parameters specific to PAHs, the researchers also considered the uncertainty associated with precipitation or advection (the horizontal mass motion of the atmosphere).
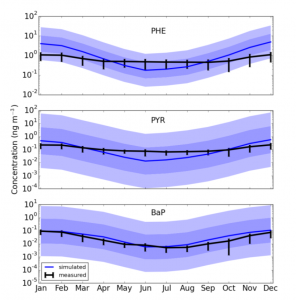
Measured (black) and simulated (blue) monthly concentrations for different PAHs, where the shaded regions mark one and two standard deviations for the uncertainty distribution. Source: Environmental Science and Technology (Pike-Thackray et al. 2015)
Notably, Pike-Thackray et al. modified their models to be consistent with experimental observations. The researchers also compared the different strategies and amount of computational power needed for different modelling approaches, and claimed that their methods offer “a significant advantage over traditional model parameter sensitivity tests” because of how they “quantify the relative importance of each parameter, as well as account for their interactions in the model system”.
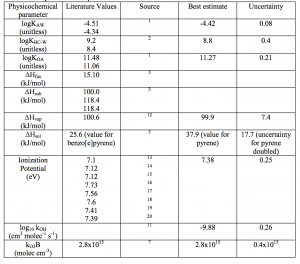
Best estimates, uncertainties, and literature values for various physical and chemical parameters associated with a specific PAH. Source: Environmental Science and Technology (Pike-Thackray et al. 2015)
Overall, this research reveals which parameters cause the greatest uncertainty in modelling the concentration and transport of PAHs in the atmosphere. My opinion is that this research is extremely interesting and worthwhile because targeting these parameters could allow the development of better environmental models and predictions, which could in turn influence both government regulation and commercial use of POPs. Furthermore, the work presented in Pike-Thackray’s thesis is an interesting example of how chemistry, environmental science, statistics and mathematics can all intersect and be applied towards a real-world issue.
— Jessica Li
References
- United States Environmental Protection Agency. Persistent Organic Pollutants: A Global Issue, A Global Response. https://www.epa.gov/international-cooperation/persistent-organic-pollutants-global-issue-global-response (accessed Mar 22, 2019).
- United States Environmental Protection Agency. DDT – A Brief History and Status. https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status(accessed Mar 22, 2019).
- The University of Maine. DDT in Alaska meltwater poses cancer risk for people who eat lots of fish. https://umaine.edu/news/blog/2018/12/06/ddt-in-alaska-meltwater-poses-cancer-risk-for-people-who-eat-lots-of-fish/(accessed Mar 22, 2019).
- Schenker, U.; Scheringer, M.; Sohn, M. D.; Maddalena, R. L.; McKone, T. E.; Hungerbühler, K. Using information on uncertainty to improve environmental fate modeling: A case study on DDT. Env. Sci Technol 2009, 43, 128–134.
- Qureshi, A.; MacLeod, M.; Hungerbuhler, K. Quantifying uncertainties in the global mass balance of mercury. Glob. Biogeochem Cycles 2011, 25.
- Kim K.; Shen, D.E.; Nagy, Z.K.; Braatz, R.D. Wiener’s Polynomial Chaos for the Analysis and Control of Nonlinear Dynamical Systems with Probabilistic Uncertainties. IEEE Control Systems Magazine, 2013, 33, 5.
- Thackray, C.P.; Friedman, C.L.; Zhang, Y.; Selin N.E. Quantitative Assessment of Parametric Uncertainty in Northern Hemisphere PAH Concentrations. Env. Sci Technol 2015, 49, 15, 9185–9193.
- Pike-Thackray, C.M. An uncertainty-focused approach to modeling the atmospheric chemistry of persistent organic pollutants. Ph.D. Dissertation, Massachusetts Institute of Technology, Cambridge, MA, 2016.