Some metals such as nickel, aluminum and steel are ubiquitous in our daily lives, and can be found in coinage, cookware, bridges, and more. Other metals, known as “precious metals”, are rarer and more expensive—but if you’ve ever owned a smartphone or taken medication, then you’ve likely benefitted from them as well.
An average iPhone contains approximately 0.034 grams of gold and 0.34 grams of silver, as well as smaller amounts of rare Earth elements such as yttrium, terbium, and neodymium. Precious metals are also used in the large-scale syntheses of commercial drugs. A common example is palladium, which catalyzes “cross-coupling” reactions—in which two molecules are coupled together—used to prepare Losartan (to treat high blood pressure) and Diflunisal (to treat fever and pain).
Unfortunately, the use of precious metals in the electronic and pharmaceutical applications is expensive and sometimes unsustainable. For example, neodymium is used to make permanent magnets used in cellphones and electric cars—yet according to Boston University’s Julie Klinger, the demand for neodymium exceeds its supply by “about 3,000 tons a year”. Because rare metal deposits are often found in only a few countries, scarcity can also result from political or economic disruptions. Furthermore, a review in Sustainable Materials and Technologies claims that “rare earth magnets are […] not being particularly sustainable in terms of their mining and refinement.”
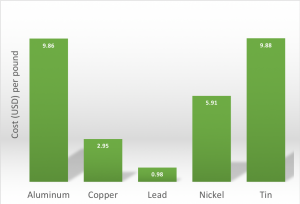
Closing prices for base metals on
Mar 1, 2019. Data from InfoMine.
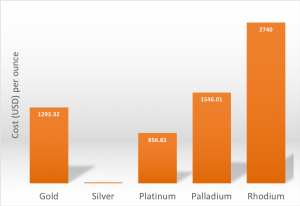
Closing prices for precious metals on Mar 1, 2019. Data from InfoMine
In February 2018, Toyota announced that it replaced 20% of the neodymium in the permanent magnets used for electric car motors with cheaper metals. Compared to neodymium, which costs about $100 per kilogram, lanthanum and cerium cost $5 to $7 per kilogram. Most metals are polycrystalline, and thus consist of “grains”—a continuous set of atoms making up a single crystal. To reduce the amount of neodymium without affecting properties like heat resistance or coercivity, researchers reduced grain size and made sure that neodymium was concentrated on the outside of the magnet, resulting in a two-layer structure.
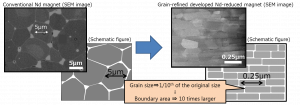
Grains in conventional and new neodymium magnets for electric cars. Source: Toyota.

Metal composition and neodymium reduction goals for magnets. Source: Toyota.
Similarly, many chemists are exploring nickel—a base, or common, metal—as an alternative for palladium-catalyzed reactions. Because nickel and palladium are in the same group of the periodic table, they have similar electron configurations and common oxidation states. Like palladium, nickel can be used to couple ring-containing molecules “commonly encountered in natural product synthesis and in the pharmaceutical sector.”
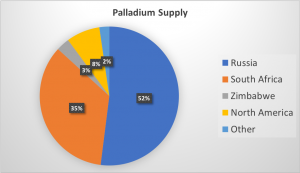
Global palladium supply by country. Data from Kitco.
One research group focused on base metal catalysis is the Chirik Group at Princeton University, who aim “not to simply replace existing catalysts but to discover new reaction chemistry that takes advantage of the unique electronic structures of […] inexpensive metal.” I agree with this research philosophy, because it acknowledges that nickel may react in ways that are different from, yet complementary to, how palladium reacts.
My opinion is that as scientists develop strategies to reduce or replace the precious metals used for various applications, more sustainable and cost-effective products will be developed—especially in the electric vehicle and drug development sectors.
— Jessica Li
References
- Nogrady, B. Your old phone is full of untapped precious metals. BBC Future. http://www.bbc.com/future/story/20161017-your-old-phone-is-full-of-precious-metals (accessed Feb 27, 2019).
- King, A.O.; Yasuda, N. Palladium-Catalyzed Cross-Coupling Reactions in the Synthesis of Pharmaceuticals. In Organometallics in Process Chemistry; Springer: Berlin, 2004; pp 205-245.
- Isaak, A. A rare metal called neodymium is in your headphones, cellphone and electric cars like Tesla’s Model 3 — and China controls the world’s supply. CNBC Tech. https://www.cnbc.com/2018/10/18/neodymium-china-controls-rare-earth-used-in-phones-electric-cars.html (accessed Feb 27, 2019.)
- Than, Ker. Critical minerals scarcity could threaten renewable energy future. Stanford EARTH. https://earth.stanford.edu/news/critical-minerals-scarcity-could-threaten-renewable-energy-future#gs.LaK2ZIMQ (accessed Feb 28, 2019).
- Widmer, J.D.; Martin, R.; Kimiabeigi, M. Electric vehicle traction motors without rare earth magnets. Sustainable Materials and Technologies 2015. 3, 7-13.
- New Toyota magnet cuts dependence on key rare earth metal for EV motors. Reuters Business News. https://www.reuters.com/article/us-toyota-magnet/new-toyota-magnet-cuts-dependence-on-key-rare-earth-metal-for-ev-motors-idUSKCN1G413F (accessed Feb 28, 2019.)
- Taleff, E.M.; Pedarazas, N.A. A New Route for Growing Large Grains in Metals. Science 2013. 341(6153), 1461-1462.
- Toyota develops new magnet for electric motors aiming to reduce use of critical rare-earth element by up to 50%. Toyota Canada Newsroom. https://media.toyota.ca/releases/toyota-develops-new-magnet-for-electric-motors-aiming-to-reduce-use-of-critical-rare-earth-element-by-up-to-50 (accessed Feb 28, 2019.)
- Hie, L. Development of Nickel-Catalyzed Cross-Coupling Reactions. Ph.D. Dissertation, University of California, Berkeley, CA, 2016.
- Base Metal Catalysis. Chirik Group. http://chemlabs.princeton.edu/chirik/research/(accessed Feb 28, 2019.)
4 responses to “Replacing rare, costly metals in electronic and pharmaceutical applications”