Throughout history, both natural and synthetic opioid drugs have been considered popular therapy for chronic pain. However, their role as effective painkillers has been challenged by the potential for desensitization and addiction. Currently, the United States (U.S.) and Canada face an opioid crisis of epidemic proportions, with overdoses and related deaths attributed to prescription opioids (POs) as well as illicit, synthetic opioids.
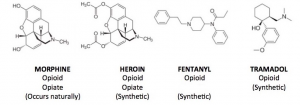
Fig 1. Chemical Structures of Opioid
This epidemic arose partly from an extensive history of over-prescribing practices. Many studies have suggested a link between PO availability and related mortality in both Canada and the U.S. Fortunately, the rise in reports connecting PO availability to mortality has been followed by a decrease in the number of opioid prescriptions, as seen in Figure 2 (2016 to 2017).
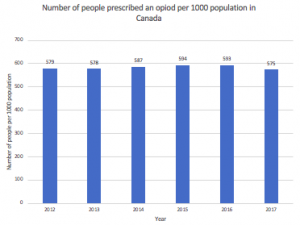
Figure 2. The Number of people prescribed an opioid. Source: CIHI
In the U.S., heroin, compared to any other single drug, is responsible for at least twice as many deaths. Alarmingly, in 2017, the number of people who have used heroin in their lifetime was over 5 million (seen in Figure 3).
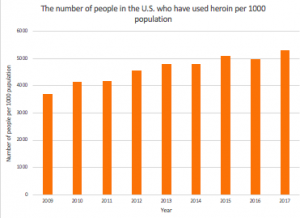
Figure 3. Statistics of people in the U.S. who have used heroin in their lifetime from 2019- 2017. Source: National Survey on Drug Use and Health
Across Canada and the U.S., current treatment for opioid use disorder predominantly rests on replacement therapy with methadone or buprenorphine, which can help reduce withdrawal symptoms and maintain abstinence. Systemically, the reinforcement of regulations for PO has been attempted in parallel with the development of anti-abuse technology.
One promising anti-abuse strategy focuses on vaccination. Interestingly, one of the earliest attempts to reduce the misuse of psychoactive substances was reported in the 1970s, when a conjugate vaccine containing morphine-like hapten was tested in animal subjects. At the time, however, the emergence of Methadone, a pharmacotherapeutic for opioid ceased further development of this vaccine. Although drug conjugate vaccine research re-emerged in the 1990s, this time focused on cocaine and nicotine, limited success in human trials challenged the clinical value of this approach to substance abuse treatment—until now.
Learning from the failures of past attempts, a team of chemist and immunologists at The Scripps Research Institute recently developed an opioid vaccine candidate that neutralizes doses of heroin without any known side effects. In essence, the vaccine stimulates the immune system to produce antibodies that bind to heroin and block it from reaching the brain thus eliminating the euphoric high.
Their study was conducted on non-human primates, specifically four monkeys that were each given three doses of the vaccine. Following vaccination, the treatment countered heroin’s effects, and it continued to provide some degree of protection for more than eight months. Given that the components of this vaccine either have been approved by the FDA or have passed safety tests previously, the researchers believe that this candidate will prove safe in humans. In order to confirm, their next step involves licensing the vaccine to an outside company and establishing a partnership for future clinical trials. As we continue refining this vaccine against heroin, its progress can pave the road for the development of even more vaccines for other opioid synthetics, thereby expanding our approaches to substance abuse treatment.
-Brina