Acid rain has become an issue in many industrial parts of the world, and has several associated risks. Acid rain, as defined by the Government of Canada, is any precipitation that has collected acidic gases or particles to gain a pH less than 5.6. This causes it to be slightly corrosive, allowing for damage to rocks and minerals and damage to plant life and ecosystems. Even buildings can suffer damage from acid rain.

A gargoyle statue that has been corroded by acid rain. Source: Wikimedia Commons
Entire ecosystems can be ravaged by acid rain given enough exposure. The image below shows a forest in the Czech Republic in 2009, with a large patch of trees that were killed. Lakes are also vulnerable to acid rain damage, as too many rains will make the lake too acidic for wildlife and vegetation to survive. A case like this even poses risk to surrounding wildlife that depend on the lake, so consequences can be widespread.

Trees killed by acid rain in a Czech Republic forest. Source: Flickr
However, some areas are more resistant to acid rain than others. Soil composition can sometimes result in the acid being neutralized before it can do too much harm. In other cases, essential compounds can be removed from the soil and plants can no longer properly uptake water.
There are several natural processes that contribute to acid rain, such as a large effect from volcanoes and a smaller one from rotting vegetation. However, these pale in comparison to the contribution from humans; namely vehicles, factories, and the burning of coal. Sulfur dioxide and nitrogen oxides are the two products of industry that cause acid rain to exist, through the chemical reactions detailed below.
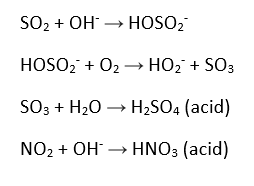
The chemical interactions in precipitation that lead to acids being formed
Incredibly rare as it is, an asteroid can even result in a massive amount of sulfur trioxide being released into the atmosphere. Japanese researchers have theorized that the asteroid that wiped out the dinosaurs 65.5 million years ago released so much sulfur trioxide into the atmosphere that a torrent of lethal acid rains directly followed its impact.
Of course, important monuments are not immune to corrosion. Limestone is one material that a lot of monuments were constructed out of, and it is also one of the most vulnerable to acid. This is significant enough that a lot of research has been done trying to develop protections for limestone structures.
If you want a visual, here is a video from Discovery with one of their staff members looking around in Washington D.C. and observing acid rain damage in some notable locations.
Scientists have done work trying to lower sulfur dioxide and nitrogen oxide release from industry, but what is the easiest solution to the threat of acid rain? Simple, cutting down on vehicle emissions and the burning of coal. Otherwise, acid rain will only continue to be a growing problem and will cause more harm to buildings, people, and ecosystems.