In the century of the internet, being able to get access to your favorite WWW whenever and wherever have become a NO.1 needs for us. To make this from dream to reality, modern technology such as WiFi, smartphone operating systems and mobile telecommunications, etc have been developed for making the internet signal approachable without the limitation on where you are and what devices you are using. But in real life, our devices are living in a complex and dangerous environment, a fragile glass won’t be able to survive through the impact of dropping and all kinds of metallic objects in your wallet are threatening the life of your phone. A company called Corning developed a glass that is tough enough to handle your daily challenges and can do even more!
Ion-exchange animation. Source
The GORILLA® glasses are immune to flaw, scratches also flexible and anti-bacterial. You may wonder now, how did they make their special glass tougher/harder? The answer is a process called ion-exchange.
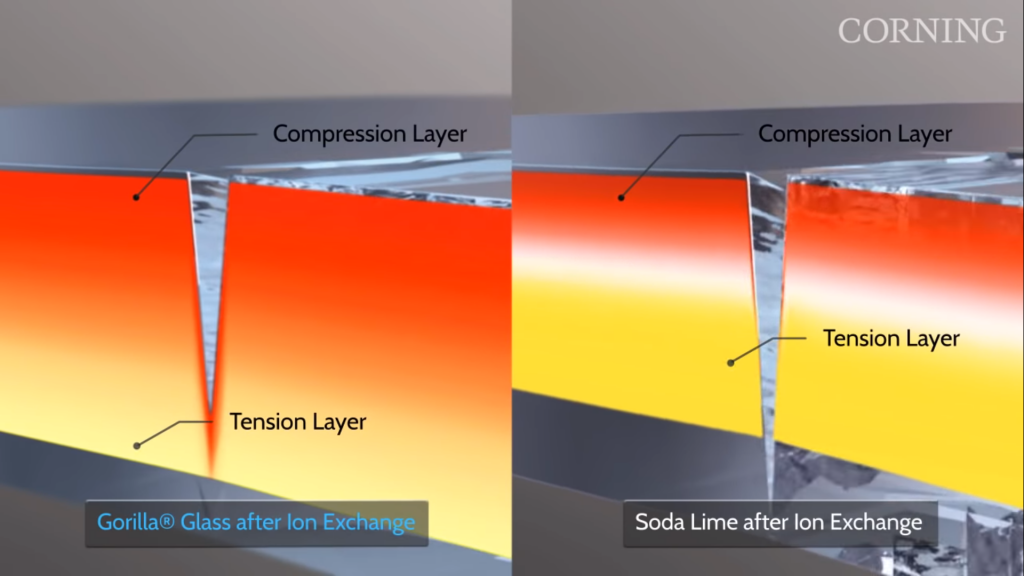
Glass under stress. Source
In a normal glass, Na+ cations are scattered throughout the structure. The normal glass is been immersed in a pool of melted potassium salt at 400°C. The high temperature enabled and P+ can take up the gap created by the leaving of Na+. The potassium cations are larger than sodium cations in size, therefore the surface of the glass is then under compression. This process can be imagined as if you have a box of basketball but you switched the basketball with the same amount but large balls. The room is the same as before so this created a tighter fit. It is this compressive stress on the surface of glasses that makes the glass resistant to scratches. This compressive stress repels all the external force that applied to the glass which means it’s tougher. Also, the compressive stress stops the extending of a scratch to the shatter of the whole glass by squeezing the small wound together!

Fusion process at isopipe. Source
There are some more technics they use to make a perfect glass fusion-draw process. The molten glasses are poured into a container called “isopipe” and the glasses fluid flow along the edge of the isopipe and meet at the bottom tip. This process ensured no flaw be introduced in forming the glass sheet.
References
Corning® Gorilla® Glass | Corning Gorilla Glass. (n.d.). Retrieved from http://www.corning.com/gorillaglass/worldwide/en/a-look-behind-corning-gorilla-glass.html
Hsu, C., Chen, G., Lin, Y., & Cheng, M. M. (2017). Anodic bonding using Gorilla Glasses. 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS). doi:10.1109/nems.2017.8017086