Scientists have recently demonstrated a faster and more efficient way of turning biofuel waste into highly valuable chemicals. These findings could have a significant impact on the economics of making fuels and other products from renewable sources.
Lignin is a component of an abundant dry plant matter called lignocellulosic biomass. This biomass source makes up the cell walls of plants and enables the upwards transport of water and provides protection against environmental stress and microbial attacks. Due to the rigidness of its structure, lignin is difficult to break down. Scientists have been trying to find methods for extracting its valuable compounds for decades, as these compounds could subsidize biofuel production, making the cost of biofuel a more competitive alternative to petroleum.
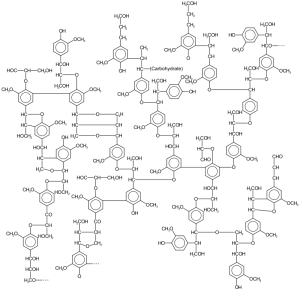
Lignin Structure. Source: Wikimedia Commons
Seema Singh and a group of researchers from Sandia National Laboratories recently studied the metabolic pathway of a soil bacteria that can naturally break down lignin. They used this metabolic pathway to come up with a method of extracting lignin’s valuable platform chemicals. The method involved genetically engineering a tobacco plant to produce large amounts of an intermediate compound called protocatechuate (PCA) as it grew. This compound was then extracted from the plant without the need to break down the lignin. Next, an engineered E. coli was added to convert the PCA into muconic acid with a yield 34% greater than previous conversion methods.
Muconic acid and pyrogallol, another product obtained using this method, are platform chemicals that are currently only derived from petroleum, and together, have a combined market value of $255.7 billion. Muconic acid can be turned into plastics, nylon, resins, and lubricants; and pyragallol has applications in cancer treatment drugs.
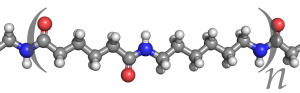
Nylon 6,6. Source: Wikimedia Commons
The next challenge in this field will be to maximize the yield and rate of production of these chemicals. In order for this method to be used on an industrial scale, large amounts of muconic acid and pyragallol must be produced in a short amount of time to compete with the current petroleum derived methods.
– Joseph Bergvinsom