Remember how recently Samsung had to discontinue their sales of Galaxy Note 7 because of their lithium battery explosion? It had been quite an issue for the world, Samsung being one of the top companies for mobile phone sales.

Battery Explosion of Samsung Galaxy Note 7, Image from flickr
However, there is no more need to worry about your phone exploding in your hands thanks to Dr. Suo and his team – they have found a way to make both safe and high-voltage lithium battery.
Originally, for high-voltage batteries (>3.0V), flammable and non-aqueous toxic electrolytes were used. They had a huge downside of causing safety and environmental concerns, especially in large-scale applications. The idealistic non-flammable and green aqueous electrolytes were too low in voltage (1.50V), reduction of water leading to hydrogen evolution being the main problem.
Suo and his team came up with another type of electrolyte called a “water-in-bisalt” (WIBS) electrolyte that fixes the problem at a wide 3.0V stability window. Suo says, “In such a concentrated environment of electrolyte, the salt anion decomposes on the anode, forming the solid electrolyte interphase (SEI) before the hydrogen evolution. SEI formation in aqueous electrolytes decreases the amount of water molecules around the Li+ molecules, reducing the electrochemical activity of water. This maximizes the full cell energy density, as Li+ is a limited resource within the battery cell.”
In short, the SEI layer formation in the WIBS electrolyte enhances the battery power and ability to repeat the cycle, as it prevents any side reactions occurring at the anode.
The team has concluded that the electrochemical coupling of electrolyte of LiMn2O4 and C-TiO2 through the carbon coating and super-concentrated aqueous electrolyte could stabilize the cycling ability of the lithium battery.
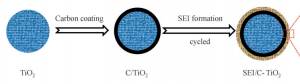
Fig 1: Carbon coated TiO2 molecule
Carbon, being catalytically inert, introduces extra kinetic barrier to water splitting; and being highly conductive, it also reduces charge transfer resistance and polarization. Also, WIBS electrolyte being high in ionic density, it terminates hydrogen evolution and enhances the formation of more protective SEI.
This is the first time to propose the new non-hazardous electrolyte battery. With this, there could be many more improvements made to commercial applications from small items like cellphones, to big items like cars. Despite this new proposed electrolyte, there still needs to be more work and refining done to the power and energy density of the new battery before it can be commercialized. Hopefully in a few years the world could be using safe, non-explosive phones.
Clair Yoon
