Figure 1. Drinking too much can cause alcohol dependence. (Courtesy Wikipedia commons)
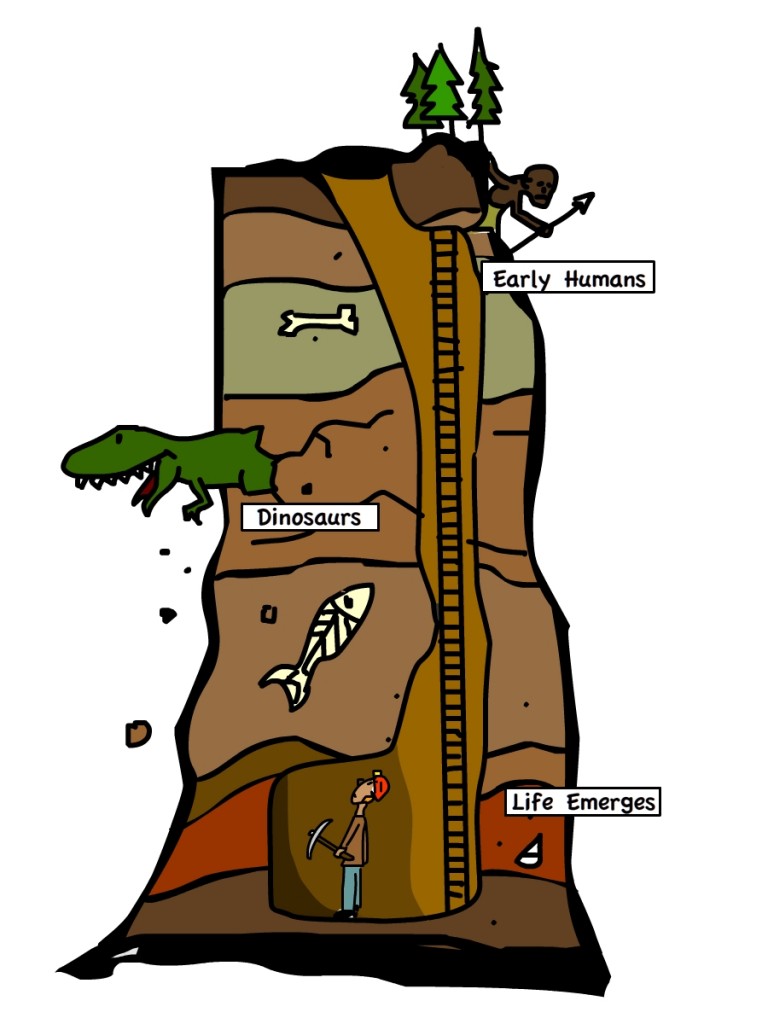
Do you know that an average of 2.5 million people die from harmful use of alcohol every year?[1] Alcohol dependence is a serious problem that can place burden on individuals and families, and even on the society. If you think that only ignorant people would allow themselves to drink excessively, you may want to think again.[2] Researchers are now suggesting that the trigger to alcohol dependence is likely due to genetic mutation.
Study led by Professor H. Thomas from Imperial College London compared two groups of mice – one group were normal, and the other group had two single base-pair point mutation in Gabrb1 gene. When the mice were given a choice between water and 10% ethanol, the latter group showed strong preference of alcohol by consuming it 85% of the time. This is equivalent to drinking one glass of wine a day! Alcohol dependence in these mice were so strong that many of them would drink sufficient alcohol to become intoxicated in an hour, and would continue to do so even after they were observed to be tipsy and had trouble moving.

Figure 2. Different types of point mutation. (Courtesy Wikipedia commons)
So why does this happen? Well, study showed that point mutation altered a series of mechanisms in the brain. To begin, Gabrb1 codes for beta1 subunit, which is an important component of GABAA receptor. Normally, GABAA receptor is activated only when GABA, a chemical messenger, is present. However, mutation to Gabrb1 causes GABAA receptor to be activated spontaneously, even when GABA is not present. These changes occur in nucleus accumbens, the brain region that controls pleasurable emotion and reward. Therefore, as more signals were sent out by GABAA receptor, mice would have increased craving for alcohol because their brains told them that alcohol consumption gave them pleasurable feelings. The study also showed not only did the mice enjoyed this feeling, they also wanted the feeling to last longer, and they did so by putting out extra physical effort, such as pushing lever for longer periods of time, in order to obtain more alcohol.

Figure 3. Location of nucleus accumbens in human brain. (Courtesy Wikipedia commons)
Professor Thomas’ study allowed researchers to gain better understanding of the mechanisms that monitor alcohol dependence in mice. Researchers believe similar mechanisms operate for humans, and are currently attempting to modify the mechanisms to human brain. GABA system is of particular interest because it controls human alcohol intake. If similar processes are found to operate in humans, this would allow doctors to screen individuals that are likely to be at risk, and ensure that early treatment can be administered.
By Kelly Liu