As I kept thinking about the water crystal pearls, I realized that I am ready to the next step – to try and understand how these superabsorbent polymers work. My first stop for information was an article in Wikipedia (thanks to the people who wrote it!). Here is what I found there:
Superabsorbent polymers (SAP) (also called slush powder) are polymers that can absorb and retain extremely large amounts of a liquid relative to their own mass.[1]
Water absorbing polymers, which are classified as hydro gels when cross-linked,[2] absorb aqueous solutions through hydrogen bonding with water molecules. A SAP’s ability to absorb water is a factor of the ionic concentration of the aqueous solution. In deionized and distilled water, a SAP may absorb 500 times its weight (from 30–60 times its own volume), but when put into a 0.9% saline solution, the absorbency drops to maybe 50 times its weight. The presence of valence cations in the solution will impede the polymers ability to bond with the water molecule.
The total absorbency and swelling capacity are controlled by the type and degree of cross-linkers used to make the gel. Low density cross-linked SAP generally have a higher absorbent capacity and swell to a larger degree. These types of SAPs also have a softer and more sticky gel formation. High cross-link density polymers exhibit lower absorbent capacity and swell, but the gel strength is firmer and can maintain particle shape even under modest pressure…
My next stop was a “How Stuff Works” web site: which also has an article on “How do polymer crystals work and why do they absorb so much water?”
I continued to poke around and found more links and of course I was brought to the Q&A about disposable diapers from About.com:
Answer: Disposable diapers contain the same chemical as astronaut ‘maximum absorbency garments”, fire-control gels, soil conditioners, those toys that grow when you add water, and floral gel. The super-absorbent chemical is sodium polyacrylate [monomer: -CH2-CH(CO2Na)- ], which was invented by scientists at Dow Chemical Company and results from polymerizing a mixture of sodium acrylate and acrylic acid.
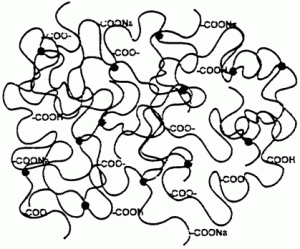
How Sodium Polyacrylate Absorbs
Superabsorbent polymers are partially neutralized polyacrylate, with incomplete cross-linking between units. Only 50–70% of the COOH acid groups have been converted to their sodium salts. The final chemical has very long carbon chains bonded with sodium atoms in the center of the molecule. When sodium polyacrylate is exposed to water, the higher concentration of water outside the polymer than inside (lower sodium and polyacrylate solute concentration) draws the water into the center of the molecule via osmosis. Sodium polyacrylate will continue to absorb water until there is an equal concentration of water inside and outside the polymer.

I enjoyed reading this blog the information provided on superabsorbent polymers is interesting.
i would enjoy the blog if it weren’t for the fact that i read the same one at http://chemistry.about.com/od/howthingsworkfaqs/f/diaper.htm
Catherine:
You are correct. This information was taken from various web sites that I indicated in the post itself. I am not an expert on these polymers, so I looked up what others had to say. If you have better sources, please let me know. If you want to see what physics activities you can do with them, read my paper in The Physics Teacher: Marina Milner-Bolotin, “Water Pearls Optics Challenges for Everybody,” The Physics Teacher 50 (1), 144-145 (2012). – THIS IS MY PAPER… Thanks for your comment. Regards, M.