Alzheimer’s disease (AD) is a type of brain disease that causes problems with thinking, memory, and behavior and leads to dementia. AD is frustratingly common among seniors over the age of 65. Approximately there are 5.8 million people in the United States are suffering; by 2050, this number will probably increase to 14 million.
How Alzheimer’s Changes the Brain. Source: https://www.youtube.com/watch?v=0GXv3mHs9AU
Although there is no current cure, an early and accurate diagnosis can help patients to access proper treatments which can slow the worsening of symptoms and improve quality of life for those suffering from AD. However, no single and efficient test can provide a reliable diagnosis. After doctors conduct interviews with patients with possible signs of symptoms, several blood tests and brain imaging are needed to rule out the other brain illnesses and confirm the diagnosis. This process may take several months, and the accuracy is only up to 75 to 85%. Researchers have been working on better and more efficient diagnostic methods. One advanced tool is Positron emission tomography (PET) scans which can detect the hallmark of abnormal protein clusters in brains and afford reliable results. However, these tests can cost thousands of dollars, and most people do not have access and never get tested.
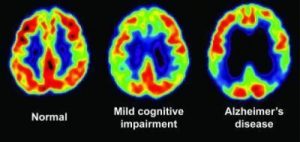
PET scan showing glucose metabolism associated with decreased cognitive function.
Source: https://www.sciencedaily.com/releases/2009/07/090714085812.htm
For decades, researchers have been on the quest to develop a blood test for AD for blood testing is the most common and affordable medical diagnostics. The exciting news is that researchers have identified blood-based biomarkers of the disease that can provide fast and accurate measurements. A biomarker is a substance whose detection indicates a particular disease; in the case of AD, the particular substance is a protein called amyloids. One significant pathological signature of AD is the appearance of clumps of abnormal amyloid protein in brains. Those clumps are made of a mixture of peptides which form from the breakdown of the amyloid precursor protein (APP). In 2010, Bateman and his colleagues from Washington University School of Medicine found that the amount of a peptide known as amyloid-b 42 (Ab42) is significantly higher in the in a patient’s blood sample. However, several follow-up studies have suggested that the number of amyloid peptides, including Ab42, increases as people grow old, so the method of detecting Ab42 is proved unhelpful. In recent years, some research shows that the ratio of Ab42 to another peptide Ab40 indicates the significant difference in the diseased brain from a cognitively normal brain. Written in Nature last year, Yanagisawa and his team from the National Center for Geriatrics and Gerontology reported that using the ratio of these two peptides as the biomarker provides highly accurate results.
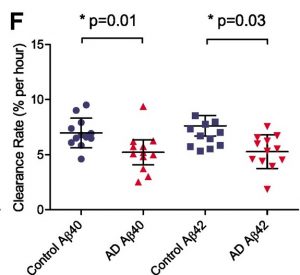
Figure 1: The clearance rate of amyloid- 40 and 42 peptides of 12 Alzheimer’s disease participants (red triangles) and 12 control (blue circles). The average clearance rate of amyloid-40 and 42 peptides is slower for AD individuals compared with cognitively normal control groups, suggesting the potential usage of these two peptides as biomarkers. Source: https://www.nature.com/articles/nature25456.
Although blood tests are not approved for commercially used yet, most researchers in the field believe that an affordable and accurate blood test for everyone will be commercially available in five years, especially when more proteins, such as neurofilament light polypeptide, are also found to be good candidates for biomarkers.
References:
National Institute on Aging. What Is Alzheimer’s Disease? https://www.nia.nih.gov/health/what-alzheimers-disease (accessed on March 21, 2019)
Alzheimer’s Association. Facts and Figures. https://www.alz.org/alzheimers-dementia/facts-figures
RadiologyInfo.org. Positron Emission Tomography-Computed Tomography. https://www.radiologyinfo.org/en/info.cfm?pg=pet (accessed on March 21, 2019)
Strimubu, K.; Tavel, J. A., Curr. Opin. HIV AIDS., 2010, 5, 463-466
O’Brien., R. J.; Wong, P. C., Annu. Rev. Neurosci. 2011, 34, 185-204
Amyloid precursor protein, Wikipedia.org, https://en.wikipedia.org/wiki/Amyloid_precursor_protein (accessed on March 21, 2019)
Mawuenyega, K. G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J. C.; Yarasheski, K. E.; Bateman, R. J., Science, 2010, 330, 1774
Arnaud, C. H., Study tests plasma biomarkers for Alzheimer’s. https://cen.acs.org/articles/96/i6/Study-tests-plasma-biomarkers-Alzheimers.html (accessed on March 21, 2019)
Nakamura, A.; Kaneko, N.; Villemagne, V.L., Kato, T.; Doecke, J.; Dore, V.; Fowler, C.; Li, Q.; Martins, R.; Rowe, C.; Tomita, T.; Matsuzaki, K.; Ishii, K.; Ishii, K.; Arahata, Y.; Iwamoto, S.; Ito, K.; Tanaka, K.; Masters, C. L.; Yanagisawa, K., Nature, 2018, 554, 249-254
Lewczuk, P.; Ermann, N.; Andreasson, U.; Schultheis, C.; Podhorna, J.; Spitzer, P.; Maler, J. M.; Kornhuber, J.; Blennow, K.; Zetterberg, H., Alzheimer’s Research & Therapy. 2018, 10