Cancer has long been a devastating condition and one that is difficult to treat, thanks to its ability to quickly propagate throughout the body. As well, the fact that cancer is contained within the body poses the issue of how to kill it off, as toxins will kill both cancer cells and normal cells. This is a problem that researchers are trying to solve by investigating a treatment named Photodynamic Therapy.
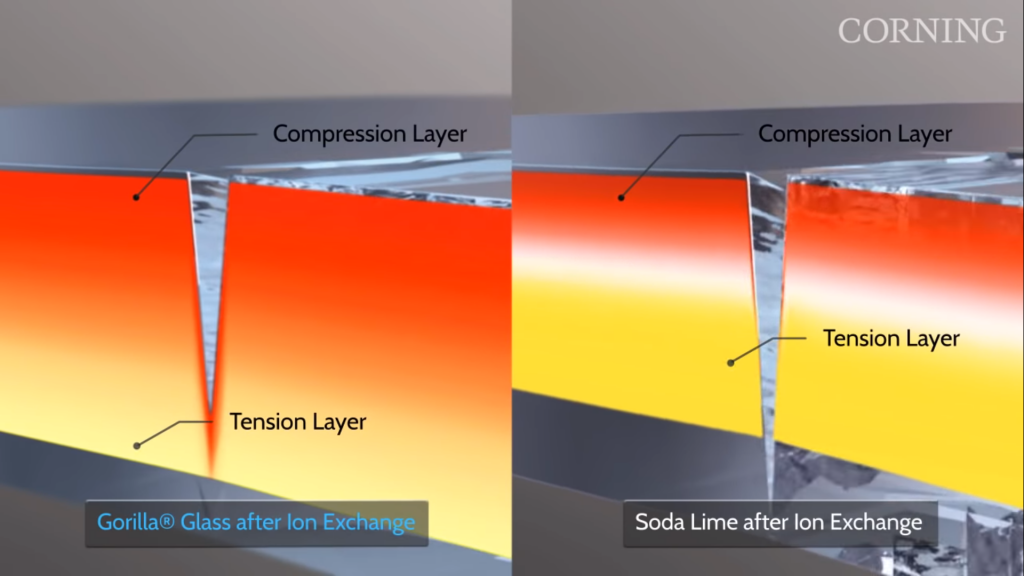
Photodynamic Therapy involves the injection of a compound called a “photosensitizer” into the bloodstream. A photosensitizer is a compound that is activated by exposure to light, and in this case produces toxic chemicals once activated. The photosensitizer is allowed to cycle throughout the bloodstream for 1-3 days, at which point it will only remain in cancer cells and not normal cells. A fiber optic cable can then be inserted into the body in order to reach the area with the tumor so that the photosensitizer can be activated.

An example of fiber optic cables used in surgery. Source: Max Pixel
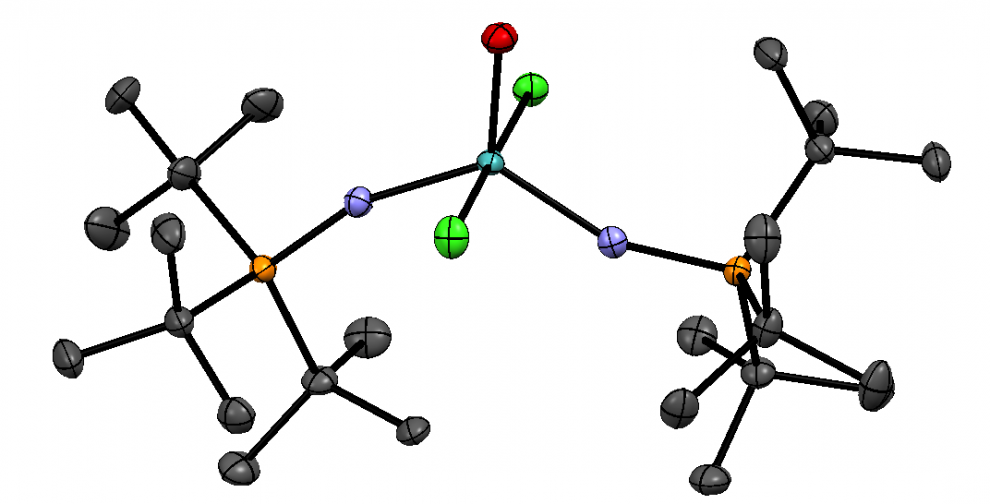
However, new research from Columbia University may present an easier way of approaching Photodynamic Therapy, one that does not require invasive procedures. The team of B. D. Ravetz et al. have discovered a method for activating photosensitizers from outside the body. They do this by using two compounds, one to absorb near-infrared (NIR) light and transfer that energy to the other, which then emits higher energy light.
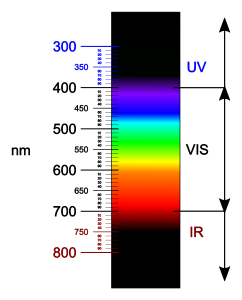
A diagram showing IR compared to visible light. Source: Wikimedia Commons
The fact that NIR light can be used for this presents some interesting applications. Unlike visible light, NIR is able to penetrate human skin and flesh quite far, meaning photosensitizers can be activated from outside the body, no incisions required! Another advantage of this is that NIR light is lower in energy than visible, meaning it has a very low risk of damaging surrounding cells.
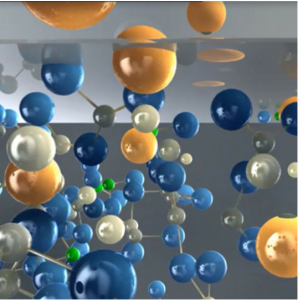
Now, how does this low energy NIR light become high energy visible light? This is done by utilizing a process called Triplet Fusion Upconversion. This same process is actually used in modern solar cells! The “sensitizer” is first excited by the NIR light, and soon loses energy from being excited and transfers it to the “annihilator.” If two energized annihilators interact, it generates a single more energized form through “triplet fusion.” This final high energy form is what then emits the visible light. Here is a brief animation that shows a similar upconversion process.
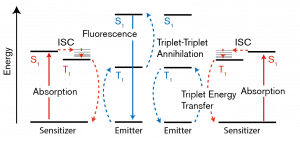
A diagram showing upconversion processes. “Emitter” is the same as Annihilator. Source: Wikimedia Commons
While this development is very promising, more testing has to be done before this can be used on humans. The toxic chemicals produced by the photosensitizer still hold a risk of killing normal cells, so tests will have to be able to control the production of these toxins. As well, the toxicity of the sensitizer and annihilator molecules will need to be evaluated too. Hopefully this procedure can be perfected in the near future, so that a safe and effective method for killing cancer can become widespread.
– Nicholas Patterson