You wake up in the morning and then press snooze on your alarm clock one more time before groggily dragging yourself out of bed to the bathroom. Quickly you brush your teeth with your electric toothbrush, then hop in the shower and lather yourself with the bottle of that fancy body wash with the microbeads in it. In the kitchen, you grab the lunch you made from the prepackaged salad mix before heading out the door. Now in your car, you turn some knobs on the dashboard to play some music on your way to work or school. In case you have lost count, you have already encountered half a dozen plastic products and it isn’t even 9 AM yet.
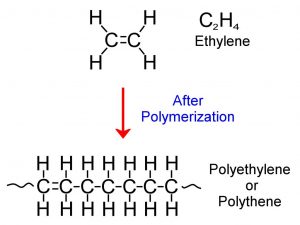
Polymerization of ethylene to form polyethylene
Source: Image © Eugbug
Plastics are the result of taking petrochemical monomers (such as ethylene) and converting them to long chain polymers. This is done through a process called polymerization which is relatively easy and cheap to do. Another process, called photo-oxidation occurs as a result of exposure of these long-chain polymers to UV radiation (from the sun) and oxygen (in the air). Essentially, this process causes plastics to become brittle and in combination with the elements (wind and water abrasion), causes the degraded plastics to break into minuscule pieces. When these pieces are between 0.1 and 1000 μm in size, they are referred to as microplastics.
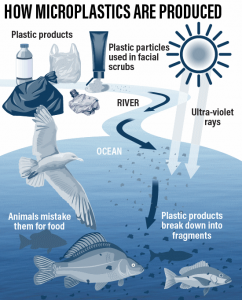
How microplastics are produced and introduced into the food cycle. Roy Cooper/The National
Due to plastics being such a cheap and omnipresent resource, there has been little incentive to recycle such products, leading to an accumulation of plastic waste in landfills and the world’s oceans. It is tragic to see seawater bodies filled with plastic, but only recently has it come into light that these microplastics are starting to make their way into our own bodies too. While seemingly obvious, microplastics have been reported in seafood, but they are also found in fertilizers and in common food items such as beer, sugar and salt. One study from last year found microplastic particles in 17 salt brands from 8 different countries. Additionally, atmospheric fallout of microplastics has also been reported, so it’s very possible we have already been inhaling and consuming microplastics.
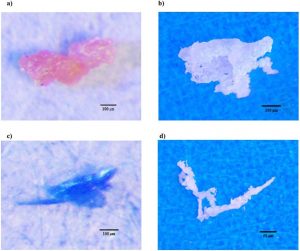
A (a) polyisoprene/polystyrene, (b) polyethylene, and (c) pigment (phthalocyanine) fragment. Image (d) is a nylon-6 filament. Source
So what can be done to mitigate the amount of plastic that is becoming ocean waste and effectively microplastics? Our daily lives and plastic products have become too intertwined to even entertain the thought of completely banning plastics worldwide. Fortunately, there have already been movements to ban especially harmful products such as microbeads found in many skin products. But, some effective steps that everyone can implement into their routines are to reduce the use of single-use plastics such as plastic straws or plastic grocery bags. The issue with single-use or “disposable” plastics is that they are difficult to recycle and thus only contribute to plastic waste. Additionally, choosing products that have less plastic packaging is also a viable way to lessen your plastic consumption. Lastly, whenever possible, recycle your plastic products so your plastic water bottle can become a new plastic water bottle and not the microplastics in our food.
The following video is a collaboration between BBC Earth Lab and Exeter University and shows how microplastics can make it through the food chain and potentially onto our plates.
~Isla