Tis the season for coughs, colds, and influenza. With winter right around the corner, flu season is nearly upon us once again, but experts believe that there may be a silver lining to this pandemic. On October 24, 2018, the U.S. Food and Drug Administration (FDA) approved of the first new class of influenza drug that is unlike anything on the U.S. market. The drug, Xofluza (the generic name is baloxavir marboxil) is a fast-acting treatment of influenza in patients at least 12 years old. The drug is taken as a single dose within 48 hours after experiencing flu symptoms, such as fatigue, fevers, and chills.
“This is the first new antiviral flu treatment with a novel mechanism of action approved by the FDA in nearly 20 years. With thousands of people getting the flu every year […] having safe and effective treatment alternatives is critical. This novel drug provides an important, additional treatment option,” said FDA Commissioner Scott Gottlieb, M.D.
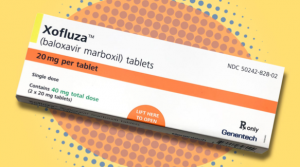
Xofluza by Catherine Roberts
Genentech, a member of the Roche group, is developing and distributing this drug to the U.S. market. The wholesale price of Xofluza will be $150, which is comparable to other antivirals including Tamiflu and Relenza. A spokesperson for Genentech stated that patients with healthcare insurance could pay as little as $30 during the pilot year Xofluza is on the market.
In two clinical trials of 1,832 patients, patients were assigned to three treatments within 48 hours of onset flu symptoms. Participants received Xofluza, a placebo pill, or another FDA-approved antiviral treatment. In both trials, participants treated with Xofluza experienced symptoms for a shorter amount of time compared to patients who were treated using a placebo pill. However, there was no difference in duration of symptoms between participants who were treated with Xofluza and those who were treated with the other antiviral. In addition, the FDA noted that adverse side effects of Xofluza were diarrhea and bronchitis.
Influenza Vaccination by Lauren Foster
So how does Xofluza work? Xofluza is an antiviral drug that inhibits viral replication, a process in which invading influenza viruses dominate the cells in the respiratory tract by making copies of themselves to spread the infection. In other words, this drug is an endonuclease inhibitor, which simply means that it specifically targets enzymes in which viruses need to multiply. According the Centers for Disease Control and Prevention (CDC), antiviral drugs can reduce the severity of symptoms and shorten the duration of the illness by about one day. Notwithstanding Xofluza’s ability to treat symptoms of the flu, the CDC states that this drug is not a substitute for the seasonal flu vaccine.
With flu season underway, there is a novel influenza treatment on the shelf for patients with the flu. The approval of Xofluza by the FDA came just in time for the upcoming flu season and experts are cautiously optimistic that Xofluza will safely and effectively treat influenza.
Written by: Jocelyn Cheng

3 responses to “New Fast-Acting, Single Dose Flu Drug is Approved by the FDA”