
A mini-Moore lab reunion at the Marine Natural Products GRC.

A mini-Moore lab reunion at the Marine Natural Products GRC.
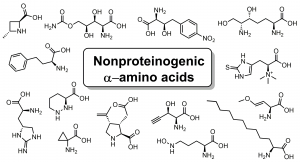 Our new review article on the biosynthesis of non-proteinogenic α-amino acids is now online at Chemical Reviews. Congrats to Jay on this work.
Our new review article on the biosynthesis of non-proteinogenic α-amino acids is now online at Chemical Reviews. Congrats to Jay on this work.
Haruka received an Overseas Postdoctoral Fellowship from The Uehara Memorial Foundation.
Congratulations, Haruka!
Welcome, Taylor!

Alyssa is the 2019 winner of the Sandra Morris and Richard Tillyer Scholarship in Chemistry.

Jay is the winner of the 2019 Brian and Jane James Graduate Scholarship in Catalysis Research
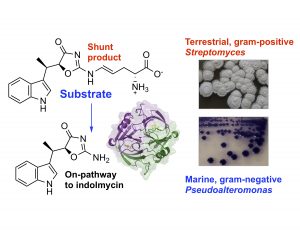
In a new paper in Nature Chemical Biology, we report that a convergent biosynthetic transformation leads to the bacterial specialized metabolite indolmycin in marine gram-negative Pseudoalteromonas luteoviolacea, when compared to a terrestrial gram-positive Streptomyces species.
Read about it here.
Congratulations to Yi-Ling, Mel, and Guiyun on this work!
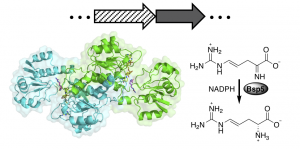 Acyclic imines are unstable in aqueous conditions, but acyclic imino acids can exhibit transient aqueous stability. Here we report the X-ray crystal structure and mechanism of Bsp5, an asymmetric acyclic imino acid reductase that intercepts acyclic imino acids produced by a partner oxidase and then converts them into D-amino acids. To read more, check out our recent paper in JACS!
Acyclic imines are unstable in aqueous conditions, but acyclic imino acids can exhibit transient aqueous stability. Here we report the X-ray crystal structure and mechanism of Bsp5, an asymmetric acyclic imino acid reductase that intercepts acyclic imino acids produced by a partner oxidase and then converts them into D-amino acids. To read more, check out our recent paper in JACS!
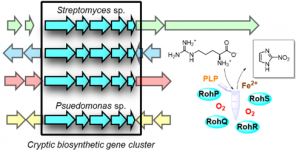 In this work reported in Angewandte Chemie, we reconstitute the biosynthetic pathway to the nitroimidazole antibiotic azomycin, revealing some interesting new enzymology. Congrats to Jay on this work!
In this work reported in Angewandte Chemie, we reconstitute the biosynthetic pathway to the nitroimidazole antibiotic azomycin, revealing some interesting new enzymology. Congrats to Jay on this work!