I want to teach you about an interesting little molecule called 2,4-dinitrophenol that is capable of uncoupling the most important cellular process in your body.
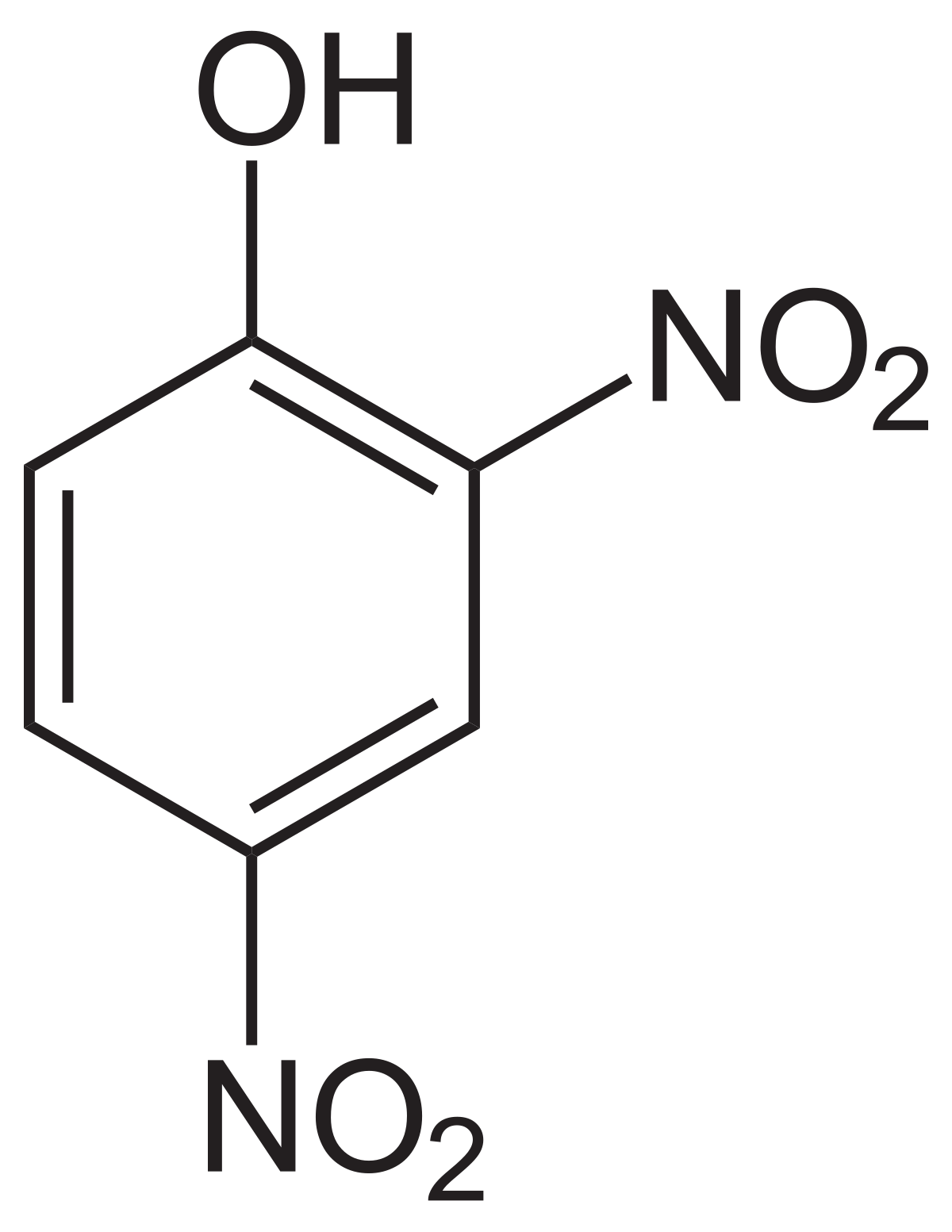
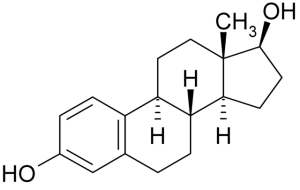
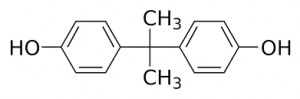
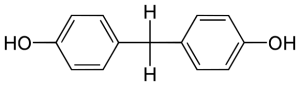
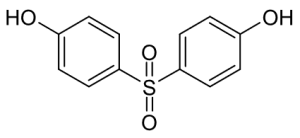
2,4-dinitrophenol is a compound that was discovered in the 1930 and for a brief time was sold as a diet pill due to its weight loss effects. When DNP is ingested orally, it finds its way into the mitochondria, positioning itself in the Inner Mitochondrial Membrane. The hydrogen of the phenol is easily deprotonated, the molecule essentially acts as a proton shuttle, moving protons from the intermembrane space to the mitochondrial matrix, by constantly getting protonated and deprotonated. This action is absolutely devastating for the cell because it is effectively uncoupling the Electron Transport Chain from Oxidative Phosphorylation.

For those of you who may need a small refresher, cellular respiration starts with the breakdown of glucose into electron carriers, which enter the mitochondria to get oxidized in the Electron Transport Chain, causing protons to be pumped into the IMS, creating a proton gradient. These protons are the driving force for the enzyme complex ATP synthase, which ultimately converts ADP to ATP in the matrix. If there is no gradient in the IMS, ATP synthase won’t be able to generate energy for the cell.
When an individual consumes DNP, the reason they lose weight is that as cellular respiration becomes less and less efficient (due to the loss of the proton gradient), the body starts burning more and more fuel in an effort to meet the energy demands of the cell, causing weight loss. So what’s the downside? Well, all the energy that should be going into ATP production ends up being lost as heat, which raises the internal core temperature to lethal levels.
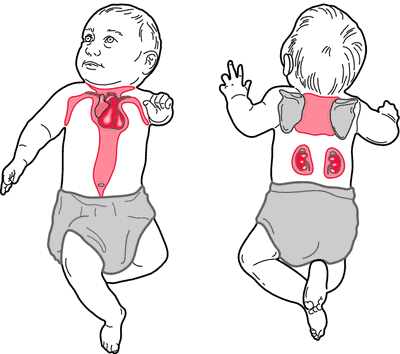
Interestingly, as bad as this sounds for you, the body actually has its own version of this molecule, which is crucial in the thermoregulation of newborns. All mammals have a type of cell called Brown Adipose Tissue (BAT) present mostly in newborns and hibernating animals, but adults also have a small quantity in their bodies. Brown fat is brown due to an increased number of mitochondria, which contain a special protein called thermogenin, located on the inner mitochondrial membrane.
Thermogenin is an uncoupling protein because its function is to uncouple the ETC from oxidative phosphorylation much in the same way DNP does. By opening a proton leak channel in the membrane and decreasing the proton gradient, heat is generated and the core temperature increases. It is especially important for the thermoregulation of infants because they don’t have any other mechanisms at their disposal to regulate their own temperature. They have little hair, not very much skeletal muscle for shivering and of course they can’t just put on a sweater!