The term heavy metal refers to any metallic chemical element that is toxic or poisonous at low concentrations. The most common heavy metals include mercury (Hg), arsenic (As), chromium (Cr), cadmium (Cd) and lead (Pb).
Heavy metals are natural components of Earth’s crust. They are non-degradable. They enter our bodies via food, drinking water and air. At very low concentrations, some heavy metals, such as copper and zinc, are essential to maintain the metabolism of the human body. However, at higher concentrations they can cause poisoning and put our lives at risk. For example, high levels of lead (II) cations can hinder brain development in children who drink from contaminated sources and cause organ damage to people of all ages. Similarly, water supplies contaminated with cadmium (II) cations cause the weakening of bones and damage our kidneys and livers.
Heavy metals are dangerous because they tend to bioaccumulate. In other words, their concentrations increase in our bodies over time. They are stored faster than they are broken down or excreted.
Heavy metals can enter a water supply by industrial and consumer waste, or from acidic rain breaking down soils and releasing heavy metals into streams, groundwater and other water sources.
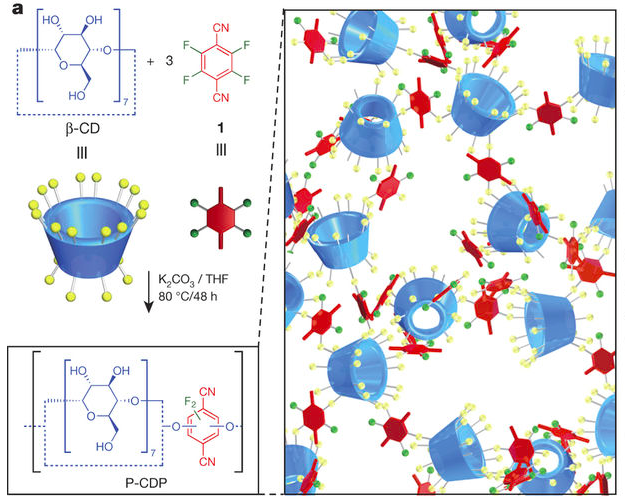
In order to remove the heavy metals from our water supplies, Weidman and co-workers functionalized nanoporous thin films of poly(acrylic acid) (PAA) with glutathione and cysteamine. Glutathione and cysteamine are peptides that exist in many organisms to counter the accumulation of heavy metal ions. For example, glutathione, which is found in many plants and animals, can bind to the metal ions and remove the ions from transport pathways within the organism.
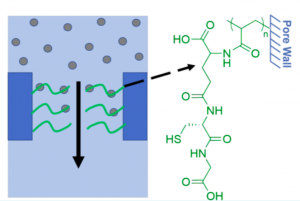
Coupling glutathione to PI−PS−PAA films can remove over 93% of heavy metal ions for drinkable water. (Source: Weidman et al., 2017)
Unlike the conventional packed bed processes, which require long residence times for effective removal of heavy metals; the nanoporous films have a lot of built-in functional groups, which allow the coupling of glutathione and cysteamine to the groups. The ready attachment of glutathione and cysteamine to the films enable efficient removal of toxic heavy metals from water supplies.
The thin films were tested with cadmium (Cd2+) and lead (Pb2+) ions. Results showed that the films could remove over 93% of the heavy metal ions. Furthermore, in mixed ion solutions, the capacity and removal rates of the films did not reduce. After repeatedly regenerating the films, the films showed no loss in capacity.
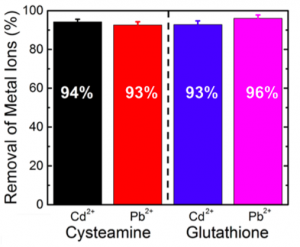
The percent of metal removed in the solution containing cysteamine-functionalized films (left) and in the solution containing glutathione-functionalized films (right). (Source: Weidman et al., 2017)
In conclusion, these thin films provide a sustainable platform for the efficient purification of lead- and cadmium- contaminated water sources to safe levels.
-Jennifer Liu-