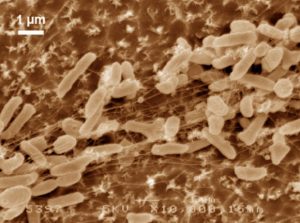
Electron Microscopy Photograph of Bacteria digesting a matrix of polyethylene molecules. Courtesy Oda.
Plastic is one the major pollutants of our oceans and lands. But for one organism, it is a tasty meal.
In a riveting study published in 2016, researchers led by Dr. Kohei Oda from the Kyoto Institute of Technology discover bacteria in soil, wastewater and plant sludge that degrade polyethylene terephthalate (PET), a major compound used in plastic products like bottles and clothing.
One of the most imperative issues we face for the future is how to create an environmentally sustainable society. A million plastic bottles are bought every minute and this number is constantly on the rise — unless we can find new technologies to recycle them at a faster rate than consumption. Wouldn’t it be great if we could throw our plastic bags into a bacteria den and voila–it would disappear?

PET is used globally in plastic products and is particularly concerning for environmentalists because of its stable chemical structure. Up until recently, the enzymatic degradation of PET was only known for a couple of fungi species. For this reason, recycling via bioremediation was not possible for plastics. However researchers in Japan screened organisms in various environments exposed to PET, such as soil and wastewater, and found that a specific bacterium was contaminated with PET particles. This organism was later isolated as Ideonella sakaienis which uses PET as its main energy and carbon source.
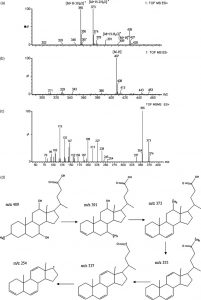
Researchers used electron microscopy to study I. sakaienis and saw that the bacterium clasp onto PET particles and release enzymes which break down the plastic into useful molecules which eventually serve as food for the bacteria. The byproduct are two environmentally friendly monomers, terephthalic acid and ethylene glycol. These results are promising and researchers hope that this solution can be applied on industrial scales to clean up deeply polluted oceans and land; however, this may not occur on timescales that are reasonable.

Plastic waste in a residential area in Los Angeles. Courtesy McDonald.
The use of bio-organisms to remedy environmental disasters is not only restricted to plastic pollutants. Some species of microbes have been observed to break down harmful compounds in oil spills.
Though this sounds good in theory, it may not work in practice. Chris Reddy, a marine chemist who worked on Vibrio and Pseudomonads species asserts that “the concept that nature will eat [oil] all up is not accurate, at least not on the time scale we’re worried about.” If microbes could digest PET or petroleum, we would not use them for our roads or plastic bottles. However, in the long run, it is a viable idea.
Microbial geochemist Samantha Joye of the University of Georgia disagrees with Reddy, and she believes that given enough time,”[microbes] are clever, they’re tough, they can basically eat nails…. The microbes have to save us again.”
Microbial remedies to the plastic problem may be a viable solution, however future work needs to focus on how to make this occur on faster timescales –time is of the essence when it comes to saving the planet.
Source:
A bacterium that degrades and assimilates poly(ethylene terephthalate)
Shosuke Yoshida et al. Science 351, 1196 (2016);