As chemistry students we usually have to defend our choice of studying chemistry against the ceaseless attacks from friends and relatives. Therefore, beside boosting the food production and saving many lives, the Haber-Bosch process is a strong argument for how chemists can change the world. Simply the Haber-Bosch process produces ammonia from gaseous nitrogen and hydrogen under high temperature and pressure (500oC, 200 atm) . Since ammonia is the key component in chemical fertilizers, the Haber-Bosch process is responsible for the annual production of the food that keeps around 7 billion people alive nowadays.
But the story of the life saving reaction does not end here, a recent modification to the Haber-Bosch process is about to write a new chapter.
In may 2017, researchers from Waseda University and Nippon Shokubai Co. Ltd. achieved a highly efficient ammonia synthesis at low temperature, with the highest yield ever reported. In the paper that was published in Chemical Science, R. Manabe et al. used an Ru catalyst and applied electric field to achieve the reported synthesis. Although the activity of Ru catalyst for ammonia synthesis in mild conditions was reported in 1972, the rate of the reaction was very slow due to the high activation energy. Here is where the finding of the paper becomes interesting.
In the presence of an electric field and hydrogen or a compound containing hydrogen to carry the ions through the reaction, the activation energy can be significantly reduced. Instead of N2 and H2 dissociation followed by N-H bonds formation, protons add up to the N2 molecule and facilitates the N-N bond cleavage. In other words, in the presence of an electric field the reaction proceeds in an associative mechanism rather than a dissociative mechanism. The addition of the proton to the N2 molecule is an example of proton hopping, in which the proton keeps jumping from one molecule to an other.
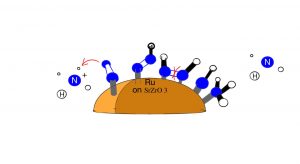
Figure (1): The steps of the associative of mechanism in the presence of an electric field
As demonstrated in the figure (1), N2 first binds to the Ru catalyst through one N atom. A proton hops non binding N. Another proton hops to the binding N, and the nonbinding N associates to the metal center. The formation of N2H molecule in an electric field releases the energy that supports the endothermic cleavage of the N-N bond. However, the figure does not include the dissociation of the H2 molecule which is catalysed by the Ru. When ammonia is produced in acidic conditions, an ammonium ion is produced which carries out the hopping process which is the first step in the figure.
This news is significant for many reasons. First, the production of ammonia consumes more the 1% of the world’s produced energy. Saving this energy would have significant economic and environmental applications. Second, the use of ammonia as hydrogen carrier for Hydrogen fuel is currently studied, and low-energy synthesis of ammonia might be required soon. Finally but most importantly, you can have a crushing argument for how chemistry can change the world!

One response to “The Life Saving Reaction: Chapter II”