“What does Red look like to you?”, and “Are you like a dog then?” are some of the many questions people suffering from colour blindness get once their genetic deficiency is uncovered. Not only are these questions woefully ignorant, but they are grossly exaggerated from the implications of the term “blindness”. This occurs since people normally attribute blindness as a condition in which an individual cannot see whatsoever, however in reality even individuals who are considered “blind” have some visual perception. In accord with this, colour-blind people are not absolutely blind to colour, with many simply having difficulty differentiating between shades of colours such as green or red. This is why colour-blindness is identified in the spectrum of Colour Vision Deficiency, or CVD for short.
Most people understand that we see things because of our eyes, but don’t actually understand how this happens, and as a result they also have difficulty understanding how CVD occurs, so a short description is provided here. In the eye, the retina is the component which receives incoming light and transmits the corresponding information to the brain resulting in colour perception. This is done by the approximately 6 million cone cells, which are categorized into the red, green, and blue types, and all individually respond to different wavelengths of light. Colour vision deficiency occurs when an individual’s eyes are unable to sense certain wavelengths of light under normal conditions due to some issue with their cone cells.
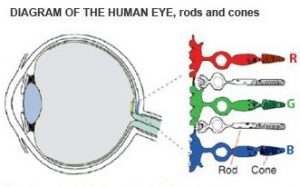
With this description, it is still difficult to visualize what it really means to be colour vision deficient, since you can’t simply switch off your cones to experience this, whereas you could close your eyes to simulate blindness. To put it simply, people with CVD, such as myself can’t see quite as wide a range of colours that a person with normal colour vision can (a great description of this can be found at this website). The outcome of this is mainly confusion for the CVD individual, with common interactions such as “Do my shoes and dress match?”, “Can you pass me the red backpack?”, or “The red line shows X, while the green line shows Y” leading to misunderstanding. Granted that such interactions can be quite humorous, the individual is often left feeling oblivious and naïve. So next time you meet someone who is colour-blind, just saying “Your eyes are pretty” will probably make their day.