Have you ever wondered how do airbags work? Do you know airbags are not inflated by any compress gas source but rather the product of a chemical reaction?? The reagent of the reaction is a really Toxic salt called sodium azide, NaN3, but is it gonna KILL you???
Under normal condition, this salt is really stable. However, if it’s heated, the salt will decompose immediately and give sodium and nitrogen gas as products.
2NaN3 → 2Na + 3N2
The equation above describes how sodium azide falls apart. It is noticeable that after the reaction, the total volume of the chemicals increased since there is only solid before reaction. And it can be calculated that under standard state condition, 130 grams of sodium azide produces about 67 liters of nitrogen gas which can inflate a normal airbag immediately (in 0.03 s!!) as the sensor detect a collision!
The following video shows how fast an airbag will expand!

You may notice that nitrogen isn’t the only product of this reaction. It produces sodium, too! Sodium is a quite reactive metal and will form sodium hydroxide (click to see the hazards) which is a strong base when reacting with water. Thus it would be hazardous if it got into your nose or eyes. To minimized the risk, sodium azide is always mixed with other chemicals which convert sodium metal into less hazardous salts. The most commonly used chemicals are potassium nitrate and silica.
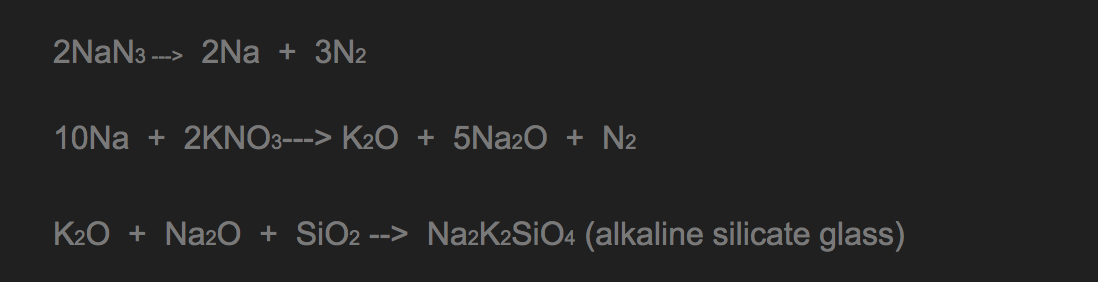
Full reaction of sodium azide by Camia and Ciara
It can be seen from the equations above that the final products of the overall reaction are nitrogen gas and Na2K2SiO4 which is a harmless alkaline silicate (click to see the hazard).
Overall, the airbag does contain toxic substances, but it is quite stable and sealed inside your car. It will NOT kill you definitely!!! When the airbag is inflated, all the toxic chemicals will be converted into harmless substances which are nitrogen gas and alkaline silicate.
Written by Xuan Wang
