Micropollutants are residue from substances, used everyday in modern society, including pharmaceuticals and personal care products (PPCPs), hormones, pesticides and industrial chemicals. According to University of Georgia Office of Research, 90% of consumed prescription drugs ultimately end up in our waste water. Micropollutants are hazardous, persistent, and not bio-degradable. They cannot be removed with conventional waste water treatment technologies. The continued release of micropollutants with wastewater effluent can cause long-term hazards such as rise of antibiotic-resistant organisms and reproductive and developmental abnormalities on sensitive species.

Wastewater treatment plant (Photo source: IWA)
Activated carbons are the most common materials used to remove organic pollutants from water. However, they have several deficiencies, including slow pollutant uptake (of the order of hours), poor removal of relatively hydrophilic micropollutants and poor regeneration performance. Since activated carbons bind to most substances through London dispersion forces, they adsorb larger molecules and non-polar molecules preferentially.
Scientists from Cornell University had synthesized porous β-cyclodextrin-containing polymers (P-CDPs) to remove the micropollutants from water. β-cyclodextrin (β-CD) is an inexpensive, sustainably produced cyclic macromolecule of glucose. It was crosslinked with rigid aromatic groups, providing a high-surface-area polymer of β-CD containing pores of 1.8–3.5 nm diameter. The resulting polymer can rapidly remove a variety of organic micropollutants with adsorption rate constants 15 to 200 times greater than those of activated carbons. In addition, the polymer can be easily and repeatedly regenerated by rinsing the polymer with methanol at room temperature while with no loss in performance. Finally, it can rapidly remove a complex mixture of organic micropollutants, including aromatic model compounds, pesticide, plastic components and pharmaceuticals, at environmentally relevant concentrations. The porous cyclodextrin-based polymers offer a rapid, flow-through water treatment.
P-CDPs were derived from nucleophilic aromatic substitution of hydroxyl (-OH) groups of β-CD by tetrafluoroterephthalonitrile (1) (Fig. a). The side groups of the P-CDPs, including fluoride (-F), cyano (-CN) and hydroxyl (-OH) groups, can bind to pollutants of different sizes and hydrophobicities through London dispersion forces and ionic, covalent and hydrogen bonds.
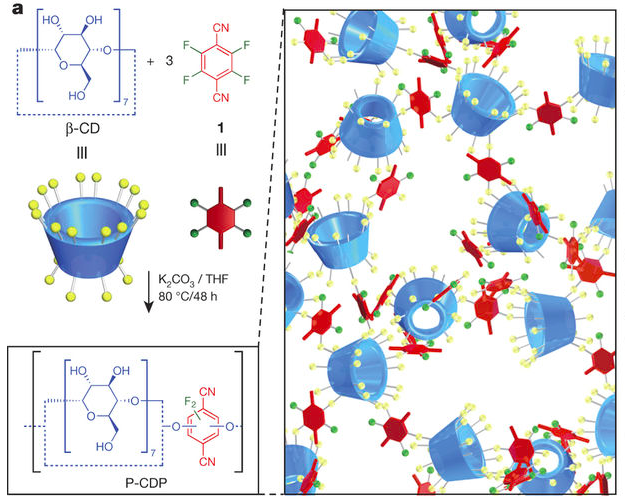
Figure a | Left, synthesis of the high-surface-area porous P-CDP from β-CD and 1. Right, schematic of the P-CDP structure. (Alsbaiee et al., 2016)
β-CD and 1 were polymerized in a suspension of potassium carbonate (K2CO3) in tetrahydrofuran (THF) at 80 °C to provide a pale-yellow precipitate, which proved to be the P-CDPs. P-CDPs obtained from 1:β-CD ratio of 3:1 exhibited the highest surface areas. A cost analysis of P-CDP indicates raw materials costs of US dollar (USD) 3.70 per kg.
Existing biological wastewater treatment plants are not specifically designed to remove micropollutants, and conventional activated carbons have poor removal performance and slow pollutant uptake. Therefore, the P-CDP, which can remove a wide range of micropollutants, presents a possible solution for wastewater treatment.
-Jennifer Liu-
