The COVID-19 pandemic disrupted thousands of clinical trials involving hundreds of thousands of participants, and billions of dollars of scientific investment. Until very recently there were no guidelines, frameworks, or standards for clinical trials that are disrupted in extenuating circumstances (e.g. pandemic, natural disaster).
An international team of scientists, patient representatives, trial investigators, methodologists and statisticians, ethicists, funders, regulators, and journal editors convened to develop the CONSERVE (CONSORT and SPIRIT Extension for RCTs Revised in Extenuating Circumstances) Statement. CONSERVE is an extension to CONSORT and SPIRIT that guide on how to report on trials and trial protocols that face important modifications in extenuating circumstances such as the COVID-19 pandemic. CONSERVE was developed using a consensus process, a rapid review, and a survey of the international trials community.
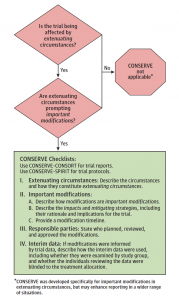
To make CONSERVE easy to implement, the statement incorporates an implementation tool and checklists tailored to trial reports and trial protocols. The checklists include 4 sections capturing extenuating circumstances, important modifications, responsible parties, and interim data analyses. The aim is to transparently report which extenuating circumstances have led to important modifications in randomized trials and trial protocols.
The CONSERVE Statement was published in JAMA and is available at the EQUATOR Network website.
