Halloween is just around the corner and can’t help but to be excited for this year’s Friday Halloween. The last time Halloween was on a Friday was in 2011, which was when I first came to UBC as a freshman. My friends and I were super hyped to celebrate this first “legal” social event and decided to go out to a bar. We ordered gin and tonic pretending to be grown-ups, like what we had seen in movies. What we did not expect was it glowing in fluorescent blue! It turned out that the bar we went to was using black lights in spirit of Halloween and that there are some physics going on behind the glass.

Source: Youtube by Jim Jesus
Tonic water used in the typical ‘gin and tonic’ drinks is carbonated soft drink containing quinine. This quinine, a natural white crystalline alkaloid, in turns contain phosphors, which is what ultimately contributes to the fluorescent glowing as it reacts with the ultraviolet (UV) light.
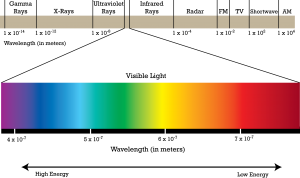
UV light is an invisible component of electromagnetic spectrum with relatively high energy. A black light, such as the one used in that bar we went to, emits UV light that can illuminate objects and materials that contain phosphors. Phosphors and the structure of quinine molecule in tonic water enable the intake of energy in the form of invisible ultraviolet light and immediately emit some of that same energy in the form of now-visible fluorescent blue light that we are seeing. This is the same principle used for your highlighters!
I found these daily-life science discoveries very amusing and still remember it to these days. In remembrance of this personal amusement, I am suggesting all of you to try this fun fluorescent drink for Halloween, or perhaps, go further to discover something even more surprising. Isn’t that what science is all about after all? Happy Halloween everyone 🙂
By Sunny Sohn