THE PROBLEM
All around the world, new COVID-19 testing centres are constantly being opened in response to the growing number of victims. These testing centres provide information about one’s diagnosis, but often through a stressful and lengthy experience. Testing centers around Canada experience wait times of up to 2 hours, and usually requires a minimum of at least 48 hours before results become available. This is a problem.
 COVID-19 Testing Centre in North Vancouver
COVID-19 Testing Centre in North Vancouver
Credit: Jane Seyd, www.nsnews.com
Besides just being tedious, this lengthy processing time has other concerns. The Director of Abbott, Norman Moore describes it:
“You’re the most infectious early on—and if we don’t have results in that timely fashion, what does it help if a molecular test comes back two weeks later?”
THE SOLUTION
This is where Moore and his team at Abbott steps in. Having already developed reliable testing tools for influenzas A&B, strep A, and respiratory syncytial virus, it was only a matter of time before COVID-19 testing apparatuses were developed. In early 2020, Abbott developed the ID Now COVID-19 Rapid Nucleic Acid Amplification Test, and launched for distribution in the US after receiving approval for emergency use from the FDA that year in March. Shortly after, Health Canada provided approval of use in October 2020.
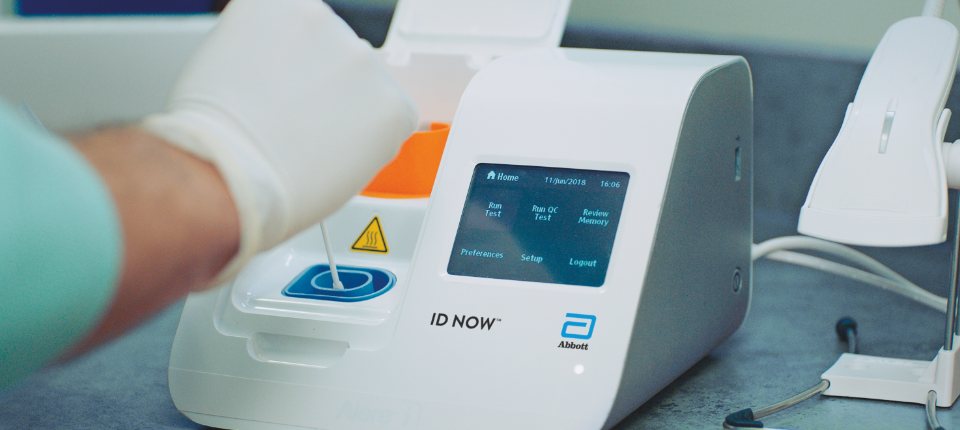 Abbott’s ID Now COVID-19 Test
Abbott’s ID Now COVID-19 Test
Credit: abbot.com
This Rapid Nucleic Acid Amplification Test takes nasal swabs from individuals suspected with COVID-19, and exposes the viral RNA packed under the outer envelope of the SARS-CoV-2 virus in an acidic solution at 56⁰ C. Just as it is written in the name, it then takes the viral RNA and amplifies it hundreds of millions of times until it is detectable by the system, and what’s more this impressive amplification process is done is a matter of minutes, and test results are returned within 13 minutes.
 A Typical Nasal Swab
A Typical Nasal Swab
Credit: U.S. Pacific Fleet, www.flickr.com
It’s normal to have some doubt when the current gold standard testing protocol in Canada (RT-PCR) takes 6-8 hours on average. We can compare the accuracy of an ID Now diagnosis to PCR lab tests by considering sensitivity and specificity.
- Sensitivity measures the proportion of people with COVID that are correctly identified
- Specificity measures the proportion of people without COVID that are correctly identified
Abbot’s clinical trial on 1003 subjects reported an average sensitivity of 93.3% and specificity of 98.4%. This is comparable to a separate meta-analysis on lab PCR testing which determined an average sensitivity of 98%, and no reported specificity. Note that these numbers are dependent on many factors, and are often higher than what is seen in the real world. This shows that ID Now can be an effective solution to rapid testing, but that results should be taken as preliminary and confirmed with other molecular assessments if results are not consistent with one’s symptoms.
This is Abbott’s response to the ‘rapidly’ changing world. They have provided frontline workers across North America with rapid testing, but whether or not they can combat COVID-19 as quickly as their tests do remains a question.
~William Lee
