Wave-swept shores can be a stressful environment for many organisms. It can be difficult for humans to truly grasp how powerful waves can be given that we are surrounded by air, which has very different properties in comparison to water. A wave travelling at a velocity of 2 m/s is roughly equivalent to a wind speed of roughly 58 m/s. Even the seemingly gentle waves can be equivalent to the force of hurricane winds. The organisms that live in these environments experience the forces of these waves every few seconds. How then do species such as kelp survive in these harsh environments, without being torn off of the rocks?
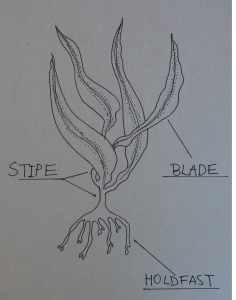
There are two traits that allow kelp to survive in these harsh environments. Kelp can develop a highly-streamlined structure in order to reduce drag. A kelp with a stream-lined structure would be one that has thin, long and flexible blades. Our researcher, Sam Starko gave a really great analogy to help conceptualize drag. He said that it is similar to the feeling one would experience when sticking your hand outside of a car window when driving. The feeling of the wind pushing against your hand, or opposite to the motion of the car is drag. Kelp can also increase tenacity or attachment force by developing a larger holdfast and thicker support tissues such as a thicker stipe. If both of these traits help to increase the chance of survival, can a species of kelp be both highly streamlined and highly tenacious?
Credit: Rachel Carr
Researchers Sam Starko and Patrick Martone from the University of British Columbia wanted to determine whether these traits, tenacity and drag, are trade-offs. A trade-off in biology exists when one trait cannot increase without the decrease in another. The study found that some kelp are highly streamlined, yet are dislodged or broken more easily (less tenacious) and some kelp experience more drag or are less streamlined yet resist greater forces before dislodging or breaking (more tenacious). Indeed, some species of kelp were found to possess intermediate traits, but no species was found to be both highly streamlined and tenacious, providing evidence for a trade-off between drag and tenacity. Tenacity was measured along the shores of Bamfield, British Columbia by attaching a spring scale to the kelp which measured the maximum force required to break the stipe or dislodge the kelp from the rock. Drag was measured in a laboratory setting using a flow tank, where water flowed against the kelp at a speed of 1 m/s. After using software to compare sequences of genes between kelp species, the results were unaffected, that is, drag and tolerance strategies were not due to species relatedness. Thus, closely related kelp can have surprisingly different morphologies (structural features).

One explanation for the existence of this trade-off is that drag and tenacity both help to increase survivorship, thus investing in both traits may be too costly. The evidence provided for this trade-off helps to explain the immense diversity seen in brown algae today.
-Allan Zhu, Rachel Carr, Robert Chandra, Sung Eun Kim (Group 3)