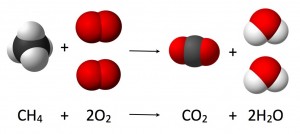
Researchers from the University of Texas at Arlington have discovered how to convert carbon dioxide and water into liquid hydrocarbon fuels in a one-step, simple and inexpensive process. Combustion is a chemical reaction that occurs between carbon and oxygen, liberating carbon dioxide and water. The equation for combustion can be shown as: CH4 + O2 –> CO2 + H20. The researchers have determined how to drive the reaction in the opposite direction by using high light intensity, concentrated heat and high pressure. The reaction takes place in a photothermalcatalytic flow reactor, operating optimally at 180-200°C and at 6 atm pressure. During the reaction, carbon dioxide and steam flow over the catalyst bed which is heated by an internal electric heater and simultaneously irradiated with UV light using lamps. The researchers used titanium dioxide as the catalyst which is advantageous because it is a cheap and abundant earth metal.
The current process produces small branched aromatics and branched linear hydrocarbons which are useful molecules for gasoline products. However, the best reaction run achieved an efficiency of 13%. Consequently, the current system is not commercially viable. Molecular oxygen is a major by-product of the reaction, detected in yields between 64-150%. Although the efficiency of the process isn’t spectacular, this is a new process and hopefully further research will result in effective modifications.
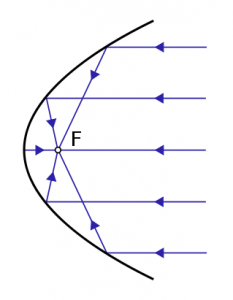
Future theoretical uses of this technology involve using solar energy to produce liquid hydrocarbons from carbon dioxide and water. Parabolic (U-shaped) mirrors can be used to concentrate the sunlight onto the catalyst. Indeed, sunlight can provide both thermal energy and photons to drive the reaction forward. However, finding an effective photo-catalyst that can absorb photons from the sun is a challenge as the current catalyst, titanium dioxide, is not able to absorb the entire visible light spectrum. The researchers argue that this process, referred to as the solar photothermalchemical alkane combustion process (SPARC) is inexpensive compared to solar biomass gasification and other related processes which produce synthesis gas (carbon monoxide and hydrogen gas). Compressing synthesis gas into usable fuels is very costly.
The results of this research have huge implications for the future of hydrocarbon fuels. As mentioned in the original research article, producing and consuming fossil fuels using this method could lead to a carbon-neutral fuel cycle. If the efficiency of this process is improved and optimized for large-scale production, arguably the greatest advantage would be that the current automotive and fuel distribution infrastructure would not have to change. However, I believe that a combination of technologies such as hydrogen fuel cells, wind power, solar energy and photovoltaic cells should be utilized appropriately to decrease global carbon dioxide emissions. For example, in certain parts of the world that have low solar insolation, solar energy is not a viable alternative but alkane reverse combustion may be a potential solution.
-Rachel Carr