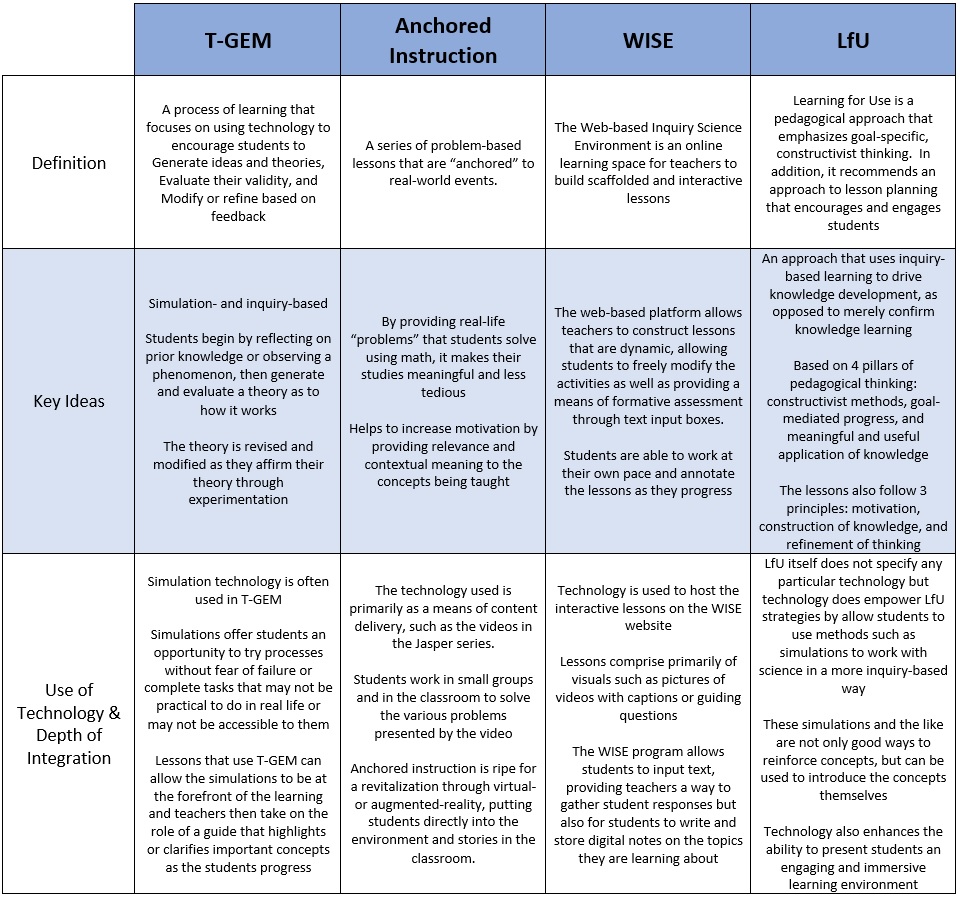
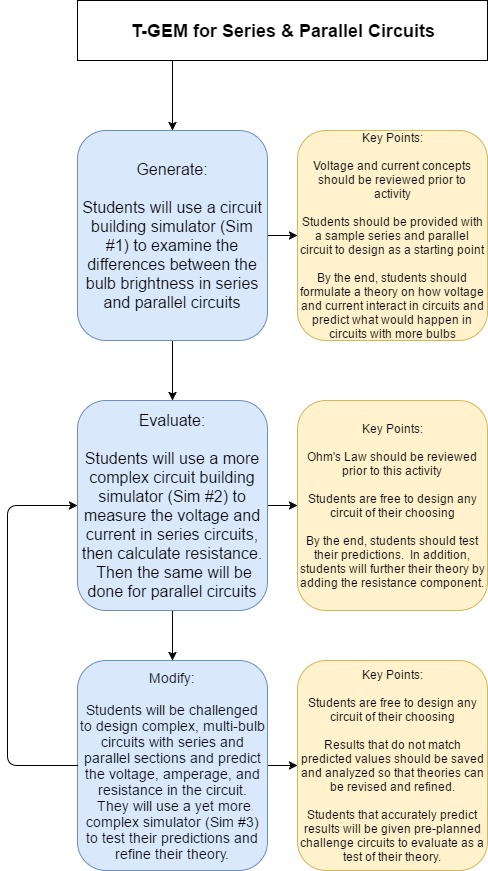
One of the major advantages to using digital simulation is the ability to aid students in visualising the invisible. In the Science 10 unit, balancing chemical equations is something that many of my students have had difficulty with. In the past, I have always fallen back on drawing and counting molecules before transitioning to more abstract calculating of number of atoms when helping students. But the process of balancing chemical equations also lends itself to a T-GEM process using PhET simulations that both help students understand the process with analogies and also to provide them a visual reference of the atoms. Moore, Chamberlain, Parson, & Perkins (2014) found benefits in using simulations in chemistry classes, noting that “students can engage with and discuss dynamic systems that provide feedback specifically designed to support student learning.” This fits neatly with the T-GEM process which plans a series of lessons that support individual learning by encouraging students to explore scientific concepts and generate theories about their relationships, then evaluate their newly constructed knowledge, and finally modify their knowledge based on the feedback from their evaluation. (Khan, 2007)
The lessons utilize three PhET simulations to start students on the concept of balancing, analogies of balanced processes, and finally the act of balancing chemical equations.
Lesson 1: Initiate Ideas – Balancing Simulation (Balance Lab)
The goal of this lesson is to prime students on the concepts of balancing. While the simulation is not strictly related to chemistry, it does visually demonstrate the concept of two parts of a single system being in balance. This will be revisited in a few lessons when students are to balance reactants and products in a chemical equation. In addition, it will serve as a starting point of a brainstorm/discussion on what other common everyday events require “balancing” and the beginning and end of the process have some quantifiable link.
Lesson 2: Generating A Theory – Reactants & Products (First with Sandwiches, then with Molecules); Additional activity – Law of Conservation of Mass Undemo
The next lesson will take the concepts of balancing and start to connect them with chemistry concepts. In particular, the simulation allows students to change the number of sandwich ingredients (reactants) and see the number of complete sandwiches (products) that can be made. During this activity, the discussion will lead towards the ideas of the ratio of reactants to products. Once students feel they have a grasp of the sandwich analogy, they can move to the molecule simulation to transfer their thinking to atoms and molecules. In addition, a live demonstration of the Law of Conservation of Mass using chemical reactants will be shown and, using the results from all three activities, students will start to formulate for themselves a concept of balanced chemical reactions.
Lesson 3: Evaluating Their Knowledge – Intro to Balancing (Introduction activity only)
As a way of evaluating their knowledge, the students will balance the three chemical equations provided in the Introduction activity. For each equation, they will first predict the adjustments necessary to balance the chemical equation. They are encouraged to using the knowledge generated from the lesson prior to complete the equations. After their prediction, they can explore both visualization tools (scale and bar graph) as well as the actual molecular diagrams as they adjust the number of each molecule to balance the equations. As part of their individualized learning, they are encourage to pick one of the three visualisation methods to use regularly. The class will finish with a sharing of various balancing strategies in order to highlight general processes used to tackle the problems.
Lesson 4: Modify Their Knowledge – Continue with Balancing (Game activity)
The Game activity has 3 difficulty levels, each with 5 questions. The students are to work through all 15 questions from Level 1-1 to Level 3-5, utilizing the strategy from Lesson 3. They are free to choose whether they want their attempts to be timed or not as a personal challenge. During this process, the teacher should be circulating and guiding students as they work through these problems, helping them re-formulate their processes as necessary if they answer a question incorrectly.
Lesson 5: Extension Of Knowledge – Working with Balanced Equations (Game activity)
The Reactants & Products simulation from Lesson 2 has a Game activity that can be used to assess and challenge student understanding. The Game provides students with a balanced equation and a specific number of either reactants or products. Using the ratio given in the balanced equation, the students have to determine the number of molecules required to complete the equation. This will challenge students to consider balanced equations from a different perspective from Lesson 4. Student understanding can be assessed formatively by engaging in discussion with them as they answer the 15 challenge questions.
References
Khan, S. (2007). Model-based inquiries in chemistry. Science Education, 91(6), 877-905.
Moore, E.B., Chamberlain, J.M., Parson, R., Perkins, K.K. (2014). PhET Interactive Simulations: Transformative Tools for Teaching Chemistry. Journal of Chemical Education, 91(8), 1191-1197