The Challenge:
Acid-base chemistry is a core section of Chemistry 11 and leads to the study of a variety of other chemical reactions. Typically, the concept of acid-base titrations is taught with traditional methods with teacher-based lectures followed by examination (Gonzalez-Gomez, Rodriquez, Canada-Canada, & Jeong, 2015). Additionally, ‘scripted’ laboratory classes can accentuate this method where students practice their observational skills and link between theory and practice. However, various misconceptions and student problems affect the ability of students to effectively learn the material. These misunderstandings can include a variety of topics such as the nature of acids and bases, and the recognition and use of acid-base chemistry (Cooper, Kouyoumdjian, & Underwood, 2016). From my own personal experience teaching acid-base titration, there is a disconnect between the laboratory work involving observations and technique and the calculations that accompany the laboratory work. Typically, students are able to complete titrations successfully in a laboratory setting and are able perform specific calculations in the classroom. However, they often struggle when the two concepts are joined or completed together. As proposed below, the use of T-GEM cycle might help alleviate some of these issues.
T-GEM Cycle:
Briefly, the T-GEM cycle involves three levels of instructional strategies (Khan, 2007):
- Compiling information and generating a relationship
- Evaluating the relationship
- Modifying the relationship
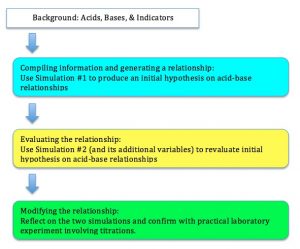
The propose T-GEM cycle for the acid-base titration lessons would involve the teacher initially providing a minimal amount of background information. This would include introducing the concepts of acids, bases, and indicators.
The first level of T-GEM involves compiling information and generating a relationship. Students would be introduced to the first simulation (see below). This simulation allows students to manipulate the amount of NaOH in the Erlenmeyer flask and virtually perform a titration to the equivalence point. Students would be tasked with determining a general relationship between the concentration of base and acid as the titration proceeds.
Following the introductory simulation on titrations, students are then asked to revaluate the relationship using a second simulation (see below). This second simulation is more involved as it allows greater control and manipulation of variables (type of reaction, specific acids, specific bases, and verification of calculation). For example, students can be tasked with finding a more specific relationship between variables and perform calculations to verify results.
The final teacher strategy involves students modifying and summarizing their initial relationship based on their observations from the second simulation. Also, students would need to solve a new case. In my classes, I would then integrate the technical lab work for students to attempt and further confirm their hypothetical relationships.
The strategies are summarized below:
Digital Tech:
Simulation #1
Simulation #2
References:
Cooper, M., Kouyoumdjian, H., & Underwood, S. (2016). Investigating students’ reasoning about acid-base reactions. Journal of Chemical Education, 93(10), 1703-1712.
Gonzalez-Gomez, D., Rodriguez, D., Canada-Canada F., & Jeong, J. (2015) A comprehensive application to assist in acid-base titration self-learning: An approach for high school and undergraduate students. Journal of Chemical Education, 92(5), 855-863.
Khan, S. (2007). Model-based inquiries in chemistry. Science Education, 91(6), 877-905.
Hi Darren,
I really like how you laid out your lesson and gave explanations as to how they can help students who struggle with misconceptions or who are unable to synthesize the titrations and calculations when they are brought together. I often find this sort of challenge to be a barrier in the classroom. Students can perform part A of an assignment and part B of an assignment but they do not understand how part A and B are linked. It is often this phase of the module that is most important in order to really solidify understanding and help students apply this knowledge in real life situations. This is why I think the synthesis of the information is so important. Until synthesis occurs students have individual units of knowledge that seem to them to be unrelated. The real learning occurs when they see the relationship between the two independent parts.
As an educator, if I have only focused on part A and part B and not the synthesis it is often much harder for me to understand where their misconceptions lie. I find this to be especially true in multi-part math problems. Students can perform individual parts of the question but do not see how those parts make up the whole.
Catherine
Hi Darren!
I remember the concept of titration being a struggle for myself when I took Chemistry 11 in high school! Your analysis of the concept using the T-GEM method is very detailed! When I learned the concept, it was taught to me rather than being learned by me. That is, I felt that the knowledge of the topic was delivered from my teacher to me and there was not much of a generative aspect to it, which may have made it a challenging concept.
Hi Darren,
In the study on Le Chatelier’s principle, the chemistry teacher engaged students in scientific processes often relegated to the lab component of the course, such as generating hypotheses, making predictions, developing conclusions. The research showed that these students performed better on tests on inquiry compared to science students enrolled across 5 other universities. It was interesting then that your post raised a number of important points from the research on science education and the link between the classroom and laboratory component: Additionally, ‘scripted’ laboratory classes can accentuate this method where students practice their observational skills and link between theory and practice. However, various misconceptions and student problems affect the ability of students to effectively learn the material. These misunderstandings can include a variety of topics such as the nature of acids and bases, and the recognition and use of acid-base chemistry (Cooper, Kouyoumdjian, & Underwood, 2016). From my own personal experience teaching acid-base titration, there is a disconnect between the laboratory work involving observations and technique and the calculations that accompany the laboratory work. Engaging in the processes of science in both environments might serve to build a bridge between these sites.
Thank you too for showing us simulations that simulate the lab. (The Chemland simulations, according to their author, represent “conceptual simulations” or abstractions of large amounts of data and concepts). In your teaching sequence, you mention that: Also, students would need to solve a new case. In my classes, I would then integrate the technical lab work for students to attempt and further confirm their hypothetical relationships. If students do not confirm their relationship in the lab, can you share what might be a next step (in the classroom or lab)?
Thank you for your flowchart and research on misconceptions in acid base chemistry for those of us who wish to explore misconceptions further, Samia
Hi Samia,
If students are unable to confirm their original relationship from the simulations following the practical laboratory work, I would likely have them reevaluate where the problem with understanding first arose – any issues would likely arise from either the simulation itself or the laboratory work. As a result, I would have students revisit the simulation as well as completing another trial in the laboratory. However, I would provide a little more guidance to ensure that students make the proper connections or conclusions. If students are still struggling with the material, I would then likely review the relevant background information is fully understood and ensure a strong foundation of concepts. If problems persist, I would compartmentalize the broader topic of titrations and have students focus on smaller aspects in hopes they can piece together their learning.